Abstract
Background
Deep vein thrombosis (DVT) and pulmonary embolism (PE) together are called venous thromboembolism (VTE) and impose a high economic burden on healthcare systems. Thousands of people are hospitalized annually due to benign and treatable diseases but die due to PE; with the adoption of appropriate prevention, these deaths can be prevented.
Objective
To investigate the cost-effectiveness of using rivaroxaban versus enoxaparin in published economic analyses for prevention of VTE after total knee (TKR) or hip replacement (THR).
Method
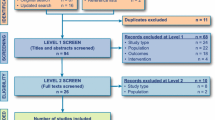
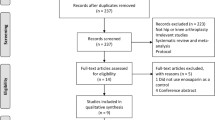
In a systematic review electronic searches were performed on various online databases, including PubMed, Web of science, Embase, Scopus, Health Economic Evaluations Database (HEED), and ProQuest. The inclusion criteria were: studies that were conducted on the cost-effectiveness of rivaroxaban versus enoxaparin for the prevention of VTE after TKR and THR; cost-effectiveness studies conducted using decision analysis models based on the economic evaluation approach; studies with available full-text papers; and studies written in English and published between 2007 and 2019. The exclusion criteria were: studies with partial cost effectiveness (such as effectiveness assessment, cost assessment, quality-of-life assessment); studies written in languages other than English; and all protocols, conference abstracts, and letters to the editor. The Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist was used to qualitatively evaluate the studies.
Results
Of a total of 537 initial studies, nine papers met the inclusion criteria. The time scope of studies ranged from 3 months to 5 years. Among the selected studies, some studies had included discount rates (n = 4) and the other studies did not utilize discount rates and were set to zero percent by default (n = 5). In all studies, direct medical costs, including costs related to the prevention, diagnosis, and treatment of VTE and PE, and management and monitoring of treatment costs were reviewed.
Conclusion
The results of this systematic review showed that using rivaroxaban in patients undergoing total knee or hip replacement reduced costs and increased quality of life. However, since most of the studies had been conducted in developed countries, it is not possible to generalize the results to developing countries. Nonetheless, given that rivaroxaban is administered orally and does not require continuous monitoring, it will be less costly for patients and health systems and is more appropriate to administer it as a thromboprophylactic drug following total knee or hip replacement surgery.


Similar content being viewed by others
References
Aslan A, Khorami R, Rezaii J, Godarzi M, Abbasi DZ. Drug updates for prevention and treatment of venous thromboembolism in orthopedic surgeries. Iranian J Cardiovasc Nurs. 2017;6(2):66–73.
Beckman MG, Abe K, Barnes K, Bartman B, Brady PJ, Hooper WC. Strategies and partnerships toward prevention of healthcare-associated venous thromboembolism. J Hosp Med. 2016;11(Suppl 2):S5.
Jha AK, Larizgoitia I, Audera-Lopez C, Prasopa-Plaizier N, Waters H, Bates DW. The global burden of unsafe medical care: analytic modelling of observational studies. BMJ Qual Saf. 2013;22(10):809–15.
Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galiè N, et al. 2014 ESC Guidelines on the Diagnosis and Management of Acute Pulmonary Embolism: The Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC) Endorsed by the European Respiratory Society (ERS). Eur Heart J Cardiovasc Pharmacother. 2014;35(43):3033–73.
Heisen M, Treur MJ, Heemstra HE, Giesen EB, Postma MJ. Cost-effectiveness analysis of rivaroxaban for treatment and secondary prevention of venous thromboembolism in the Netherlands. J Med Econ. 2017;20(8):813–24.
Health Select Committee. The prevention of venous thromboembolism in hospitalised patients. London: The Stationery Office Limited; 2005.
Centers for Disease Control and Prevention (CDC). Venous thromboembolism in adult hospitalizations-United States, 2007–2009.
Rathbun S. The surgeon general’s call to action to prevent deep vein thrombosis and pulmonary embolism. Circulation. 2009;119(15):e480–2.
Geerts WH, Pineo GF, Heit JA, Bergqvist D, Lassen MR, Colwell CW, et al. Prevention of venous thromboembolism: the seventh ACCP conference on antithrombotic and thrombolytic therapy. Chest. 2004;126(3):338–400.
Ruppert A, Lees M, Steinle T. Clinical burden of venous thromboembolism. Curr Med Res Opin. 2010;26(10):2465–73.
Cohen AT, Agnelli G, Anderson FA, Arcelus JI, Bergqvist D, Brecht JG, et al. Venous thromboembolism (VTE) in Europe. Thromb Haemost. 2007;98(10):756–64.
Beckman MG, Hooper WC, Critchley SE, Ortel TL. Venous thromboembolism: a public health concern. Am J Prev Med. 2010;38(4):S495–501.
Habibzadeh H, Mohammadi A, Safari MB, Khalkhali HR, Rezaeipour N. The effect of care plan based on nutrition and exercise on deep vein thrombosis in patients undergoing lower limb orthopedic surgery in Urmia Imam Khomeini Hospital in 1391. J Urmia Nurs Midwifery Fac. 2013;11(9):680–7.
Derman PB, Fabricant PD, David G. The role of overweight and obesity in relation to the more rapid growth of total knee arthroplasty volume compared with total hip arthroplasty. J Bone Jt Surg. 2014;96(11):922–8.
Brockbank J, Wolowacz S. Economic evaluations of new oral anticoagulants for the prevention of venous thromboembolism after total hip or knee replacement: a systematic review. Pharmacoeconomics. 2017;35(5):517–35.
Moghtadaee M, Shahhoseini GH, Farahini H, Yegane A, Rajabpour S. Dabigatran etexilate, a novel oral direct thrombin inhibitor, for preventing thromboembolic events after knee replacement arthroplasty. Tehran Univ Med J. 2012;69(11):725–9.
Monreal M, Folkerts K, Diamantopoulos A, Imberti D, Brosa M. Cost-effectiveness impact of rivaroxaban versus new and existing prophylaxis for the prevention of venous thromboembolism after total hip or knee replacement surgery in France, Italy and Spain. Thromb Haemost. 2013;110(11):987–94.
Diamantopoulos A, Lees M, Wells PS, Forster F, Ananthapavan J, McDonald H. Cost-effectiveness of rivaroxaban versus enoxaparin for the prevention of postsurgical venous thromboembolism in Canada. J Thromb Haemost. 2010;104(10):760–70.
National Center for Biotechnology Information. PubChem Database. Rivaroxaban, CID = 9875401, https://pubchem.ncbi.nlm.nih.gov/compound/Rivaroxaban. Accessed 15 May 2020.
Schellack G, Modau T, Schellack N. Clinical overview of venous thromboembolism. S Afr Fam Pract. 2016;58(1):39–45.
Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated health economic evaluation reporting standards (CHEERS) statement. Int J Technol Assess Health Care. 2013;29(2):117–22.
McCullagh L, Tilson L, Walsh C, Barry M. A cost-effectiveness model comparing rivaroxaban and dabigatran etexilate with enoxaparin sodium as thromboprophylaxis after total hip and total knee replacement in the Irish healthcare setting. Pharmacoeconomics. 2009;27(10):829–46.
Ryttberg L, Diamantopoulos A, Forster F, Lees M, Fraschke A, Björholt I. Cost-effectiveness of rivaroxaban versus heparins for prevention of venous thromboembolism after total hip or knee surgery in Sweden. Expert Rev Pharmacoecon Outcomes Res. 2011;11(5):601–15.
Duran A, Sengupta N, Diamantopoulos A, Forster F, Kwong L, Lees M. Cost effectiveness of rivaroxaban versus enoxaparin for prevention of post-surgical venous thromboembolism from a US Payer’s perspective. Pharmacoecon Open. 2012;30(2):87–101.
Zindel S, Stock S, Müller D, Stollenwerk B. A multi-perspective cost-effectiveness analysis comparing rivaroxaban with enoxaparin sodium for thromboprophylaxis after total hip and knee replacement in the German Healthcare Setting. BMC Health Serv Res. 2012;12(1):192.
McDonald H, Diamantopoulos A, Wells P, Lees M, Folkerts K, Forster F, et al. Cost-effectiveness of rivaroxaban in the prevention of venous thromboembolism: a Canadian analysis using the Ontario Ministry of Health perspective. J Med Econ. 2012;15(5):817–28.
Neves JR, Folkerts K, Umbelino S, Santos IF. Cost-effectiveness of rivaroxaban compared with enoxaparin for the prevention of venous thromboembolism in adult patients undergoing elective hip or knee replacement surgery in Portugal. Rev Port Farmacoter. 2014;6:16–23.
Yan X, Gu X, Xu Z, Lin H, Wu B. Cost-effectiveness of different strategies for the prevention of venous thromboembolism after total hip replacement in China. Adv Ther. 2017;34(2):466–80.
Fantom JN, Umar S. The World Bank’s classification of countries by income (English). Policy Research working paper; no. WPS 7528. Washington, D.C; 2016: World Bank Group
Haacker M, Hallett TB, Atun R. On discount rates for economic evaluations in global health. Health Policy Plan. 2020;35(1):107–14.
van Korlaar IM, Vossen CY, Rosendaal FR, Bovill EG, Cushman M, Naud S, Kaptein AA. The impact of venous thrombosis on quality of life. Thromb Res. 2004;114(1):11–8.
Griffin XL, McBride D, Nnadi C, Reed MR, Rossiter ND. “Don’t shoot the messengers…..”: the new NICE guidance for the prevention of venous thromboembolism in adults–fake news or a real opportunity? Bone Jt J. 2018;1136–1137.
Rodarte RR, Guimarães JA, Franco JS, Fonseca L, Nascimento V, Aramburu JP, et al. Systematic review of prophylaxis for venous thromboembolism after knee arthroplasty: enoxaparin versus rivaroxaban. Rev Col Bras Cir. 2019;46(2):e2075.
Huang HF, Li SS, Yang XT, Xie Q, Tian XB. Rivaroxaban versus enoxaparin for the prevention of venous thromboembolism after total knee arthroplasty: a meta-analysis. Medicine. 2018; 97(48).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain clinical studies or patient data.
Conflict of interest
The authors declare that they have no conflicts of interest.
Funding
This study was part of a PhD thesis supported by the Iran University of Medical Sciences (IUMS/SHMIS with Grant no: 98-2-37-15593 and with ethical code IR.IUMS.REC.1398.534).
Rights and permissions
About this article
Cite this article
Rashki Kemmak, A., Abutorabi, A. & Alipour, V. Economic Evaluation of Rivaroxaban Versus Enoxaparin for Prevention of Venous Thromboembolism After Total Knee Replacement and Total Hip Replacement: A Systematic Review. Clin Drug Investig 40, 715–725 (2020). https://doi.org/10.1007/s40261-020-00940-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-020-00940-4