Abstract
Background
Several second-generation antipsychotics (SGAs) are available in long-acting injectable (LAI) formulations.
Objective
To systematically review the effects of the two formulations, Monohydrate and Lauroxil, of Aripiprazole LAI in patients with schizophrenia and bipolar disorder during an acute episode or during maintenance treatment.
Methods
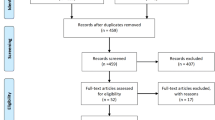
On September 18, 2018, we adopted the following search strategy: (aripiprazole OR OPC-14597 OR Abilify) AND (long-acting OR depot OR LAI OR once monthly OR prolonged release OR monohydrate OR lauroxil) on PubMed, Cochrane, Scopus, CINAHL, PsycINFO, and Web of Science to identify randomised controlled trials. Furthermore, we searched the ClinicalTrials.gov site for possible additional studies.
Results
We included 28 papers dealing with randomised assignment of aripiprazole LAI formulations in schizophrenia and bipolar disorder in survival studies after stabilisation, in acute studies, and in head-to-head comparisons. Both monohydrate and lauroxil formulations reduced relapses/recurrences with respect to comparators (placebo or 50 mg once-monthly monohydrate) and improved symptomatology in acute schizophrenia.
Limitations
Only a small number of studies were included in our review, with widely overlapping samples. While a high proportion of studies were wholly or partly industry-sponsored, their outcomes do not appear to have been affected.
Conclusion
Aripiprazole LAI may to be efficacious in reducing relapse of schizophrenia and bipolar disorder in the long term in stabilised patients and in improving symptoms of schizophrenia during its acute phase, with both monohydrate and lauroxil formulations showing efficacy.

Similar content being viewed by others
References
Stevens GL, Dawson G, Zummo J. Clinical benefits and impact of early use of long-acting injectable antipsychotics for schizophrenia. Early Interv Psychiatry. 2016;10(5):365–77.
Leucht S, Heres S. Epidemiology, clinical consequences, and psychosocial treatment of nonadherence in schizophrenia. J Clin Psychiatry. 2006;67(Suppl 5):3–8.
Leucht C, Heres S, Kane JM, Kissling W, Davis JM, Leucht S. Oral versus depot antipsychotic drugs for schizophrenia-a critical systematic review and meta-analysis of randomised long-term trials. Schizophr Res. 2011;127(1–3):83–92.
Amano T, Matsubayashi H, Momiyama T, Ishihara K, Todo N, Sasa M. Antagonizing effects of a novel antipsychotic quinolinone derivative (OPC-14597) on dopaminergic inhibition of neuronal activities in the nucleus accumbens. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19(1):105–16.
Fujikawa M, Nagashima M, Inoue T, Yamada K, Furukawa T. Partial agonistic effects of OPC-14597, a potential antipsychotic agent, on yawning behavior in rats. Pharmacol Biochem Behav. 1996;53(4):903–9.
Cañive JM, Lewine JD, Edgar JC, Davis JT, Miller GA, Torres F, Tuason VB. Spontaneous brain magnetic activity in schizophrenia patients treated with aripiprazole. Psychopharmacol Bull. 1998;34(1):101–5.
United States Food and Drug Administration. Approval package for 21-436/S-005 & S-008 & 21-713/S-003. Rockville (Maryland): U.S. Food and Drug Administration; 2005.
European Medicines Agency. Committee for Medicinal Products for Human Use (CHMP): Assessment report EMA/557557/2014. Procedure No. EMEA/H/C/000471/II/0101. London: EMA, 24 July 2014. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/000471/WC500174667.pdf. Accessed 11 Sept 2018.
Tessler L, Goldberg I. Crystal structures of aripiprazole, a new anti-psychotic drug, and of its inclusion compounds with methanol, ethanol and water. J Incl Phenom Macrocycl Chem. 2006;55:255–61.
Nahata T, Saini TR. D-optimal designing and optimization of long acting microsphere-based injectable formulation of aripiprazole. Drug Dev Ind Pharm. 2008;34(7):668–75.
Nahata T, Saini TR. Formulation optimization of long-acting depot injection of aripiprazole by using D-optimal mixture design. PDA J Pharm Sci Technol. 2009;63(2):113–22.
Kane JM, Sanchez R, Perry PP, Jin N, Johnson BR, Forbes RA, McQuade RD, Carson WH, Fleischhacker WW. Aripiprazole intramuscular depot as maintenance treatment in patients with schizophrenia: a 52-week, multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2012;73(5):617–24.
United States Food and Drug Administration (US FDA). Abilify Maintena™ (aripiprazole) for extended release injectable suspension, for intramuscular use: prescribing information. Revised on February 2013. http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/202971s000lbl.pdf. Accessed 11 Sept 2018.
Mallikaarjun S, Kane JM, Bricmont P, McQuade R, Carson W, Sanchez R, Forbes RA, Fleischhacker WW. Pharmacokinetics, tolerability and safety of aripiprazole once-monthly in adult schizophrenia: an open-label, parallel-arm, multiple-dose study. Schizophr Res. 2013;150(1):281–8.
Raoufinia A, Baker RA, Eramo A, Nylander AG, Landsberg W, Kostic D, Larsen F. Initiation of aripiprazole once-monthly in patients with schizophrenia. Curr Med Res Opin. 2015;31(3):583–92.
Kirschbaum KM, Müller MJ, Malevani J, Mobascher A, Burchardt C, Piel M, Hiemke C. Serum levels of aripiprazole and dehydroaripiprazole, clinical response and side effects. World J Biol Psychiatry. 2008;9(3):212–8.
United States Food and Drug Administration (US FDA). Abilify Lauroxil (aripiprazole) for extended release injectable suspension, for intramuscular use: prescribing information. 10-02-2015. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&varApplNo=207533. Accessed 11 Sept 2018.
Rohde M, Mørk N, Håkansson AE, Jensen KG, Pedersen H, Dige T, Jørgensen EB, Holm R. Biological conversion of aripiprazole lauroxil—an N-acyloxymethyl aripiprazole prodrug. Results Pharma Sci. 2014;4:19–25.
Turncliff R, Hard M, Du Y, Risinger R, Ehrich EW. Relative bioavailability and safety of aripiprazole lauroxil, a novel once-monthly, long-acting injectable atypical antipsychotic, following deltoid and gluteal administration in adult subjects with schizophrenia. Schizophr Res. 2014;159(2–3):404–10.
Ehret MJ, Davis E, Luttrell SE, Clark C. Aripiprazole Lauroxil NanoCrystal® Dispersion Technology (Aristada Initio®). Clin Schizophr Relat Psychoses. 2018;12(2):92–6.
Hard ML, Wehr AY, Sadler BM, Mills RJ, von Moltke L. Population pharmacokinetic analysis and model-based simulations of aripiprazole for a 1-day initiation regimen for the long-acting antipsychotic aripiprazole lauroxil. Eur J Drug Metab Pharmacokinet. 2018;43(4):461–9.
Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, Moher D, Tugwell P, Welch V, Kristjansson E, Henry DA. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008.
Miller SA. PICO Worksheet and Search Strategy. Los Angeles (CA): National Center for Dental Hygiene Research, University of Southern California; 2001.
Cohen J. Statistical power analysis for the behavioral sciences. New York: Routledge; 1988.
Cohen J. A power primer. Psychol Bull. 1992;112(1):155–9.
Sawilowsky S. New effect size rules of thumb. J Mod Appl Stat Methods. 2009;8:467–74.
Higgins, J.P.T., Green, S. (eds.). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. London, UK: The Cochrane Collaboration, 2011. http://www.handbook.cochrane.org. Accessed 11 Sept 2018.
Kane JM, Zhao C, Johnson BR, Baker RA, Eramo A, McQuade RD, Duca AR, Sanchez R, Peters-Strickland T. Hospitalization rates in patients switched from oral anti-psychotics to aripiprazole once-monthly: final efficacy analysis. J Med Econ. 2015;18(2):145–54.
Taylor DM, Sparshatt A, Amin F, Osborne I, Dzahini O, Hughes G, Fischetti C. Aripiprazole long-acting injection—a mirror image study of its effects on hospitalisation at one year. J Psychopharmacol. 2017;31(12):1564–9.
Kane JM, Sanchez R, Baker RA, Eramo A, Peters-Strickland T, Perry PP, Johnson BR, Tsai LF, Carson WH, McQuade RD, Fleischhacker WW. Patient-centered outcomes with aripiprazole once-monthly for maintenance treatment in patients with schizophrenia: results from two multicenter, randomized, double-blind studies. Clin Schizophr Relat Psychoses. 2015;9(2):79–87.
Peters-Strickland T, Zhao C, Perry PP, Eramo A, Salzman PM, McQuade RD, Johnson BR, Sanchez R. Effects of aripiprazole once-monthly on symptoms of schizophrenia in patients switched from oral antipsychotics. CNS Spectr. 2016;21(6):460–5.
Kane JM, Sanchez R, Zhao J, Duca AR, Johnson BR, McQuade RD, Eramo A, Baker RA, Peters-Strickland T. Hospitalisation rates in patients switched from oral anti-psychotics to aripiprazole once-monthly for the management of schizophrenia. J Med Econ. 2013;16(7):917–25.
Fleischhacker WW, Sanchez R, Perry PP, Jin N, Peters-Strickland T, Johnson BR, Baker RA, Eramo A, McQuade RD, Carson WH, Walling D, Kane JM. Aripiprazole once-monthly for treatment of schizophrenia: double-blind, randomised, non-inferiority study. Br J Psychiatry. 2014;205(2):135–44.
Ishigooka J, Nakamura J, Fujii Y, Iwata N, Kishimoto T, Iyo M, Uchimura N, Nishimura R, Shimizu N; ALPHA Study Group. Efficacy and safety of aripiprazole once-monthly in Asian patients with schizophrenia: a multicenter, randomized, double-blind, non-inferiority study versus oral aripiprazole. Schizophr Res. 2015;161(2–3):421–8.
Calabrese JR, Sanchez R, Jin N, Amatniek J, Cox K, Johnson B, Perry P, Hertel P, Such P, Salzman PM, McQuade RD, Nyilas M, Carson WH. Efficacy and safety of aripiprazole once-monthly in the maintenance treatment of bipolar I disorder: a double-blind, placebo-controlled, 52-week randomized withdrawal study. J Clin Psychiatry. 2017;78(3):324–31.
Fleischhacker WW, Sanchez R, Johnson B, Jin N, Forbes RA, McQuade R, Baker RA, Carson W, Kane JM. Long-term safety and tolerability of aripiprazole once-monthly in maintenance treatment of patients with schizophrenia. Int Clin Psychopharmacol. 2013;28(4):171–6.
Fleischhacker WW, Baker RA, Eramo A, Sanchez R, Tsai LF, Peters-Strickland T, Perry PP, McQuade RD, Johnson BR, Carson WH, Kane JM. Effects of aripiprazole once-monthly on domains of personal and social performance: results from 2 multicenter, randomized, double-blind studies. Schizophr Res. 2014;159(2–3):415–20.
Kane JM, Peters-Strickland T, Baker RA, Hertel P, Eramo A, Jin N, Perry PP, Gara M, McQuade RD, Carson WH, Sanchez R. Aripiprazole once-monthly in the acute treatment of schizophrenia: findings from a 12-week, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2014;75(11):1254–60.
Calabrese JR, Sanchez R, Jin N, Amatniek J, Cox K, Johnson B, Perry P, Hertel P, Such P, McQuade RD, Nyilas M, Carson WH. Symptoms and functioning with aripiprazole once-monthly injection as maintenance treatment for bipolar I disorder. J Affect Disord. 2018;227:649–56.
Calabrese JR, Sanchez R, Jin N, Amatniek J, Cox K, Johnson B, Perry PP, Hertel P, Such P, McQuade RD, Nyilas M, Carson WH. The safety and tolerability of aripiprazole once-monthly as maintenance treatment for bipolar I disorder: a double-blind, placebo-controlled, randomized withdrawal study. J Affect Disord. 2018;241:425–32.
Meltzer HY, Risinger R, Nasrallah HA, Du Y, Zummo J, Corey L, Bose A, Stankovic S, Silverman BL, Ehrich EW. A randomized, double-blind, placebo-controlled trial of aripiprazole lauroxil in acute exacerbation of schizophrenia. J Clin Psychiatry. 2015;76(8):1085–90.
Ismail Z, Peters-Strickland T, Miguelez M, Baker RA, Hertel P, Eramo A, Jin N, Perry P, Sanchez R, McQuade RD, Kane JM. Aripiprazole once-monthly in the treatment of acute psychotic episodes in schizophrenia: post hoc analysis of positive and negative syndrome scale Marder factor scores. J Clin Psychopharmacol. 2017;37(3):347–50.
Nasrallah HA, Newcomer JW, Risinger R, Du Y, Zummo J, Bose A, Stankovic S, Silverman BL, Ehrich EW. Effect of aripiprazole lauroxil on metabolic and endocrine profiles and related safety considerations among patients with acute schizophrenia. J Clin Psychiatry. 2016;77(11):1519–25.
Citrome L, Du Y, Risinger R, Stankovic S, Claxton A, Zummo J, Bose A, Silverman BL, Ehrich EW. Effect of aripiprazole lauroxil on agitation and hostility in patients with schizophrenia. Int Clin Psychopharmacol. 2016;31(2):69–75.
Potkin SG, Risinger R, Du Y, Zummo J, Bose A, Silverman B, Stankovic S, Ehrich E. Efficacy and safety of aripiprazole lauroxil in schizophrenic patients presenting with severe psychotic symptoms during an acute exacerbation. Schizophr Res. 2017;190:115–20.
Targum SD, Risinger R, Du Y, Pendergrass JC, Jamal HH, Silverman BL. Effect of patient age on treatment response in a study of the acute exacerbation of psychosis in schizophrenia. Schizophr Res. 2017;179:64–9.
Citrome L, Risinger R, Cutler AJ, Du Y, Zummo J, Nasrallah HA, Silverman BL. Effect of aripiprazole lauroxil in patients with acute schizophrenia as assessed by the Positive and Negative Syndrome Scale-supportive analyses from a Phase 3 study. CNS Spectr. 2018;23(4):284–90.
McEvoy JP, Risinger R, Mykhnyak S, Du Y, Liu CC, Stanford AD, Weiden PJ. Durability of therapeutic response with long-term aripiprazole lauroxil treatment following successful resolution of an acute episode of schizophrenia. J Clin Psychiatry. 2017;78(8):1103–9.
Naber D, Hansen K, Forray C, Baker RA, Sapin C, Beillat M, Peters-Strickland T, Nylander AG, Hertel P, Andersen HS, Eramo A, Loze JY, Potkin SG. Qualify: a randomized head-to-head study of aripiprazole once-monthly and paliperidone palmitate in the treatment of schizophrenia. Schizophr Res. 2015;168(1–2):498–504.
Cuomo I, Kotzalidis GD, de Persis S, Piacentino D, Perrini F, Amici E, De Filippis S. Head-to-head comparison of 1-year aripiprazole long-acting injectable (LAI) versus paliperidone LAI in comorbid psychosis and substance use disorder: impact on clinical status, substance craving, and quality of life. Neuropsychiatr Dis Treat. 2018;14:1645–56.
Girardi P, Del Casale A, Rapinesi C, Kotzalidis GD, Splendori F, Verzura C, Trovini G, Sorice S, Carrus D, Mancinelli I, Comparelli A, De Filippis S, Francomano A, Ballerini A, Marcellusi A, Mennini FS, Ducci G, Sani G, Pompili M, Brugnoli R. Predictive factors of overall functioning improvement in patients with chronic schizophrenia and schizoaffective disorder treated with paliperidone palmitate and aripiprazole monohydrate. Hum Psychopharmacol. 2018;33(3):e2658.
De Hert M, Eramo A, Landsberg W, Kostic D, Tsai LF, Baker RA. Efficacy and safety of aripiprazole once-monthly in obese and nonobese patients with schizophrenia: a post hoc analysis. Neuropsychiatr Dis Treat. 2015;11:1299–306.
Potkin SG, Loze JY, Forray C, Baker RA, Sapin C, Peters-Strickland T, Beillat M, Nylander AG, Hertel P, Nitschky Schmidt S, Ettrup A, Eramo A, Hansen K, Naber D. Relationship between response to aripiprazole once-monthly and paliperidone palmitate on work readiness and functioning in schizophrenia: a post hoc analysis of the QUALIFY study. PLoS One. 2017;12(8):e0183475 (Correction: PLoS One. 2017;12(11):e0188416).
Potkin SG, Loze JY, Forray C, Baker RA, Sapin C, Peters-Strickland T, Beillat M, Nylander AG, Hertel P, Steen Andersen H, Eramo A, Hansen K, Naber D. Reduced sexual dysfunction with aripiprazole once-monthly versus paliperidone palmitate: results from QUALIFY. Int Clin Psychopharmacol. 2017;32(3):147–54.
Potkin SG, Loze JY, Forray C, Baker RA, Sapin C, Peters-Strickland T, Beillat M, Nylander AG, Hertel P, Nitschky Schmidt S, Eramo A, Hansen K, Naber D. Multidimensional assessment of functional outcomes in schizophrenia: results from QUALIFY, a head-to-head trial of aripiprazole once-monthly and paliperidone palmitate. Int J Neuropsychopharmacol. 2017;20(1):40–9.
Pandina G, Lane R, Gopal S, Gassmann-Mayer C, Hough D, Remmerie B, Simpson G. A double-blind study of paliperidone palmitate and risperidone long-acting injectable in adults with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(1):218–26.
Li H, Rui Q, Ning X, Xu H, Gu N. A comparative study of paliperidone palmitate and risperidone long-acting injectable therapy in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(4):1002–8.
Fleischhacker WW, Gopal S, Lane R, Gassmann-Mayer C, Lim P, Hough D, Remmerie B, Eerdekens M. A randomized trial of paliperidone palmitate and risperidone long-acting injectable in schizophrenia. Int J Neuropsychopharmacol. 2012;15(1):107–18.
Fu DJ, Bossie CA, Sliwa JK, Ma YW, Alphs L. Paliperidone palmitate versus oral risperidone and risperidone long-acting injection in patients with recently diagnosed schizophrenia: a tolerability and efficacy comparison. Int Clin Psychopharmacol. 2014;29(1):45–55.
Takekita Y, Koshikawa Y, Fabbri C, Sakai S, Sunada N, Onohara A, Nishida K, Yoshimura M, Kato M, Serretti A, Kinoshita T. Cognitive function and risperidone long-acting injection vs. paliperidone palmitate in schizophrenia: a 6-month, open-label, randomized, pilot trial. BMC Psychiatry. 2016;16:172.
Acknowledgements
We gratefully acknowledge the contribution of the Librarians of the School of Medicine and Psychology of Sapienza University, Ms. Mimma Ariano, Ms. Felicia Proietti, Ms. Ales Casciaro, Ms. Teresa Prioreschi, and Ms. Susanna Rospo for rendering precious bibliographical material accessible, as well as our Secretary Lucilla Martinelli for her assistance during the writing of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work has not been supported by any funding.
Conflict of interest
Drs. Girardi and Brugnoli received research grants from Otsuka during 2016–2017; Dr. Girardi received educational and research funds from Janssen, Lilly, Angelini, and Springer. All other authors have no relevant affiliations or financial involvement with any organisation or entity with a financial interest in, or financial conflict with the subject matter or materials discussed in the manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rapinesi, C., Kotzalidis, G.D., Mazzarini, L. et al. Long-Acting Injectable (LAI) Aripiprazole Formulations in the Treatment of Schizophrenia and Bipolar Disorder: a Systematic Review. Clin Drug Investig 39, 713–735 (2019). https://doi.org/10.1007/s40261-019-00801-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-019-00801-9