Abstract
Background and Objectives
Lilly insulin glargine (LY IGlar; Basaglar®) and the reference insulin glargine product (IGlar; Lantus®) are basal insulin glargine analogs with identical amino acid sequence and similar pharmacological profiles. ELEMENT 5, a Phase 3, prospective, randomized, multinational, two-arm, active-controlled, open-label, parallel-design study in type 2 diabetes mellitus (T2DM) patients (N = 493) showed similar efficacy and safety profiles with LY IGlar and IGlar. This study reports results from India (N = 100) and East Asia (N = 134) subpopulations.
Methods
Patients from India and East Asia (Korea and Taiwan) with T2DM who were insulin naïve (glycated hemoglobin (HbA1c) ≥ 7.0% and ≤ 11.0%) or on basal insulin (HbA1c ≤ 11.0%) were randomized to receive LY IGlar or IGlar along with oral antihyperglycemic medications (OAMs) for 24 weeks. Patients were instructed to self-titrate from the starting dose by 1 unit/day until fasting blood glucose (FBG) ≤ 5.6 mmol/L (100 mg/dL) was achieved. The key outcome was HbA1c change from baseline to Week 24.
Results
Within-group least-squares mean (LSM) decrease (baseline to Week 24) in HbA1c was similar between treatments. The upper limit of confidence interval (CI) for treatment difference was below the defined 0.4% noninferiority margin in India (LY IGlar: − 0.83%; IGlar: − 0.62%; difference [95% CI] − 0.21 [− 0.70, 0.28]) and East Asia (LY IGlar: − 1.28%; IGlar: − 1.26%; difference [95% CI] − 0.02 [− 0.34, 0.30]) subpopulations. Results of other efficacy and safety endpoints at Week 24 were similar between treatments in both subpopulations. LSM self-monitored FBG levels were similar between treatments at all visits in both subpopulations except at Week 24 in the India subpopulation (LY IGlar: 5.65 [0.10] mmol/L or 101.8 [1.86] mg/dL; IGlar: 5.18 [0.10] mmol/L or 93.3 [1.75] mg/dL; p = 0.002).
Conclusion
Efficacy and safety profiles of LY IGlar and IGlar, in combination with OAMs, were similar in India and East Asia subpopulations. This was consistent with the ELEMENT 5 total population.
Clinical Trial Registration
NCT02302716.
Similar content being viewed by others
1 Introduction
Diabetes is currently a major health concern. The International Diabetes Federation (IDF) estimates that the global prevalence of diabetes will increase from 425 million people in 2017 to 629 million people in 2045. Type 2 diabetes mellitus (T2DM) accounts for more than 90% of all patients with diabetes [1].
Insulins play an important role in the treatment of T2DM [2]. A recent consensus report by the American Diabetes Association and the European Association for the Study of Diabetes highlights the importance of initiation of basal insulin in patients with T2DM who fail to attain glycemic targets with non-insulin therapeutic options [3]. Insulin glargine was the first long-acting insulin analog to be used in clinical practice [4]. It was designed to, as far as possible, meet the body’s basal insulin requirements [5]. Basal insulin glargine has an extended duration of action; its effect lasts for nearly 24 h with a more uniform concentration-time profile. This allows for convenient once-daily (QD) dosing coupled with reduced risks of hypoglycemia [6, 7]. In addition, inter-subject variability of blood insulin concentration associated with basal insulin glargine is less than that with neutral protamine Hagedorn (NPH) [8]. Thus, basal insulin glargine is being widely considered globally as an important treatment option for T2DM.
The therapeutic landscape of diabetes has witnessed an emergence of multiple biosimilar insulins in the past few years, which is opening up more treatment options for patients with diabetes [9]. Studies that have evaluated biosimilar insulins against reference insulin products are very limited [10]. Lilly insulin glargine (LY IGlar; Basaglar®, Eli Lilly and Company, Indianapolis, IN, USA, and Boehringer Ingelheim, Ingelheim am Rhein, Germany) is the first biosimilar insulin to have received approval in the highly regulated markets; it was approved in the EU and Japan in the year 2014 followed by approval as the first follow-on insulin in the USA [11]. It has recently received approval from the regulatory authorities in Korea, Taiwan, and India. Thus, LY IGlar has set a precedent for the development of biosimilar insulins. As part of the LY IGlar development program, we conducted the pivotal ELEMENT 1 and ELEMENT 2 studies for approval of LY IGlar in the EU, USA, and Japan. We also conducted the ELEMENT 5 study comparing the efficacy and safety of LY IGlar with the reference insulin glargine product (IGlar; Lantus®, Sanofi Aventis, Paris, France) to provide additional data for some regulatory agencies [12]. The patients who participated in the ELEMENT 5 study were predominantly Asians (Indians and East Asians; 47.5%) and Whites (45.6%). Results from the ELEMENT 5 study have further substantiated the similar efficacy and safety profiles of LY IGlar and IGlar when given in combination with oral antihyperglycemic medications (OAMs) in T2DM patients [13]. There are some underlying differences in the pathophysiology and clinical features of T2DM between Asians and their Western counterparts. Asians are at a risk of developing diabetes at a lower body mass index (BMI) than other ethnicities. Greater visceral obesity, higher insulin resistance, impaired insulin secretion, and smaller β-cell mass are common risk factors for diabetes in Asians [14, 15]. In addition, there is a more conservative approach to insulin treatment in Asia, often resulting in suboptimal glycemic control [16, 17]. Taking these factors into consideration and coupled with the fact that 60% of the world’s diabetic population is in Asia [18], we feel that results from the India and East Asia subpopulations from the ELEMENT 5 study may be of interest. Here, we present post hoc results for the India and East Asia subpopulations from the ELEMENT 5 study.
2 Patients and Methods
2.1 Study Design and Patients
ELEMENT 5, a Phase 3, prospective, randomized, multinational, multicenter, two-arm, active-controlled, open-label, parallel-design study was conducted in patients with T2DM in India, Korea, Taiwan, Russia, Turkey, the USA, and Puerto Rico. The study compared the efficacy and safety profiles of LY IGlar and IGlar in patients with T2DM. The study comprised a screening visit (about 2 weeks before randomization), a randomization visit (Week 0), treatment period spanning 24 weeks (visits at Weeks 2, 4, 8, 12, 16, 20, and 24), and a 4-week post-treatment follow-up period (Fig. S1). The study was conducted in accordance with the International Council for Harmonization Guidelines for Good Clinical Practice and the Declaration of Helsinki following approval by the pertinent ethics boards. All patients provided written informed consent prior to entering the study.
The study included adults with T2DM with a BMI ≤ 45 kg/m2, who were either insulin naïve (glycated hemoglobin [HbA1c] ≥ 7% and ≤ 11.0%) or on basal insulin (HbA1c ≤ 11.0%) and receiving at least two OAMs. Permissible basal insulin interventions included QD IGlar, NPH insulin, or insulin detemir either QD or twice daily (BID) for a minimum of 90 days before entering the study. Usage of more than QD IGlar, any insulin other than the aforementioned ones in the last 30 days or glucagon-like-peptide receptor agonists, or biosimilar IGlar in the last 90 days was not permitted. Patients who were receiving chronic systemic glucocorticoid therapy for more than 14 consecutive days at the time of screening or had received such therapy within 4 weeks before screening were excluded from the study. Other major exclusion criteria were significant cardiac or gastrointestinal disorders, history or diagnosis of cancer or human immunodeficiency virus, excessive insulin resistance (total insulin dose ≥ 1.5 U/kg), one or more than one episode of severe hypoglycemia in the past 6 months, and known hypersensitivity to IGlar or its excipients.
Randomization was stratified by country, HbA1c levels (< 8.5% vs. ≥ 8.5%), sulfonylurea usage (yes/no), and use of basal insulin prior to entering the study (yes/no), and was managed using an interactive web-response system. Treatments (LY IGlar or IGlar) were administered QD at around the same time in combination with prescribed dosages of OAMs for 24 weeks. The starting dose for insulin-naïve patients was 10 U/day. For patients receiving basal insulin prior to entering the study, the starting dose was either the same as the QD dose or 80% of the BID dose. Patients were instructed to self-titrate by 1 U/day until fasting blood glucose (FBG) levels were ≤ 5.6 mmol/L (100 mg/dL).
2.2 Outcomes
The study aimed to compare the efficacy and safety profiles of LY IGlar and IGlar in patients with T2DM. The key objective of the subpopulation analyses was to assess if the change in HbA1c from baseline to Week 24 was similar between LY IGlar and IGlar and to evaluate if the confidence interval (CI) of the difference in treatment is below the set + 0.4% noninferiority margin. A similar evaluation was done by a second gated test in the subgroup of patients who were on IGlar prior to entering the study. Additional efficacy outcomes included changes in HbA1c after Weeks 4, 8, 12, 16, and 20; proportion of patients achieving HbA1c levels < 7% and ≤ 6.5%; 7-point self-monitored blood glucose (SMBG) assessments (before each meal, 2 h after morning/mid-day meals, at bedtime, and at 3:00 h); basal insulin dose (U/day and U/kg/day) weight; and BMI. HbA1c assessments were done by Covance (Indianapolis, IN, USA) at regional centers. Glucometers (Accu-Chek Performa®; Roche, Indianapolis, IN, USA) were provided for SMBG measurements.
The satisfaction level of patients was assessed using the Insulin Treatment Satisfaction Questionnaire (ITSQ). The ITSQ is a validated measure of treatment satisfaction for patients with diabetes who are taking insulin. It has 22 items, which patients are instructed to rate on a 7-point scale. In addition to an overall score, the items that make up the five domains of satisfaction are categorized as: inconvenience of regimen (five items), lifestyle flexibility (three items), glycemic control (three items), hypoglycemic control (five items); and insulin delivery device (six items). Individual patient domain scores and overall scores were calculated using pre-defined formulae. A higher score indicated better treatment satisfaction [19].
Information on adverse events (AEs) was collected at each visit. Adverse events were defined as medical untoward occurrences either newly reported or worsening in severity after randomization. These were recorded as treatment-emergent AEs. The investigator determined if the treatment-emergent AEs were related to the study drug. Injection-site AEs were analyzed based on responses of patients to the Skin Evaluation Questionnaire and Insulin Questionnaire: Injection Sites. It considered factors associated with injection (pain, pruritus, and rash) and injection site characteristics (including lipohypertrophy, hemorrhage, or induration). Immunogenicity measures included proportion of patients with detectable anti-insulin antibodies and the antibody levels (percentage binding). Immunogenicity samples were analyzed at Eurofins Pharma Bioanalytics (St Charles, MO, USA).
Hypoglycemic events were evaluated as annualized rates (hypoglycemic events/person/year) and incidences (proportion of patients with at least one hypoglycemia event). Hypoglycemic events were defined as events when blood glucose levels were ≤ 3.9 mmol/L (70 mg/dL) or signs or symptoms associated with hypoglycemia. All such events were categorized as total hypoglycemia. A hypoglycemic event was considered nocturnal hypoglycemia if it occurred after bedtime and before the first morning meal. Any hypoglycemic episode that required assistance from another person was categorized as severe hypoglycemia and was reported as a serious AE (SAE).
2.3 Statistical Analyses
The full analysis set, which included all patients who received at least one dose of the drug that they were assigned to, was used for all analyses of efficacy and safety measures. Unless otherwise stated, all analyses of treatment effects used the two-sided alpha level of 0.05 and corresponding 95% confidence intervals (CIs). The key endpoint (change in HbA1c from baseline to Week 24), self-monitored FBG, basal insulin dose, and weight change from baseline were analyzed using the mixed-model repeated-measures method. The analyzed outcomes were the dependent variables in each model. Treatment (LY IGlar, IGlar), the baseline value, basal insulin at entry (yes/no), sulfonylurea use (yes/no), visit, and interaction between visit and treatment were fixed effects. Baseline HbA1c was a covariate and patient was a random effect. Hypoglycemia rates over the 24-week period were analyzed using a Negative Binomial model to make comparisons between treatment and control groups. The outcome was the number of episodes, and the independent variables were sulfonylurea usage at screening, basal insulin status at study entry, HbA1c at baseline, and the treatment arm, with log (exposure in days/365.25) as an offset variable.
Fisher’s exact test or Pearson’s Chi-square test was used for comparison of categorical variables whereas comparisons of continuous variables were performed using Student’s t test, unless otherwise stated. Rates of hypoglycemic episodes per person per year (total, nocturnal, documented symptomatic, and severe) were analyzed using a Negative Binomial model, with the number of episodes as dependent variable, the treatment arm as covariate, and the log of the observation period as offset variable. All analyses were evaluated using SAS® version 9.2 (SAS Institute, Cary, NC, USA). The analyses were not powered to make statistical comparisons between the two subpopulations or with the ELEMENT 5 total population.
3 Results
3.1 Patients
The ELEMENT 5 total population included 493 patients [13] (India subpopulation: 133 screened and 100 randomized; East Asia subpopulation: 153 screened and 134 randomized). The most common reason for screen failures was baseline HbA1c levels being beyond the limits defined in the inclusion criteria. Investigator’s decision and withdrawal of consent by patients were the more common reasons for discontinuation (Fig. S2). Baseline demographics and clinical characteristics were generally similar between the two treatment arms for both India and East Asia subpopulations and consistent with the trends observed in the ELEMENT 5 total population (Table 1). In the India subpopulation, the mean (standard deviation) HbA1c levels were 8.73% (1.12%) and 8.52% (1.14%) in LY IGlar and IGlar, respectively; in the East Asia subpopulation, the corresponding values were 8.68% (1.01%) and 8.57% (1.04%), respectively. In the India subpopulation, 46.9% (LY IGlar) and 45.1% (IGlar) were insulin naïve. In the East Asia subpopulation, 29.9% (LY IGlar) and 34.3% (IGlar) were insulin naïve. A majority of patients in both subpopulations were taking sulfonylurea in combination with one or more OAMs at baseline. Among patients who were on basal insulin prior to entering the study, most patients in the India and East Asia subpopulations were receiving IGlar.
3.2 Glycemic Responses
Both LY IGlar and IGlar showed a statistically significant reduction in HbA1c from baseline at the end of 24 weeks in India and East Asia subpopulations (p < 0.001 within each treatment group). These findings were similar to those in the ELEMENT 5 total population (Fig. 1). The decrease in HbA1c from baseline to Week 24 for LY IGlar was comparable with that of IGlar in both subpopulations (India: p = 0.389; East Asia: p = 0.905). The LSM (least-squares mean) difference (95% CIs) in HbA1c change from baseline to Week 24 between the two treatment arms was − 0.21% (− 0.70%, 0.28%) in India and − 0.02% (− 0.34%, 0.30%) in East Asia subpopulations. The upper limit of the 95% CI was below the set noninferiority margin of + 0.4%. Thus, the key objective was met in both the subpopulations. This observation was consistent with the ELEMENT 5 total population (Table 2).
HbA1c levels over 24 weeks. a India subpopulation; b East Asia subpopulation; c ELEMENT 5 total population. Values presented are LSM (standard error). Analyses are based on mixed-model repeated-measures. Data points are slightly offset along the x axis to avoid overlapping error bars. HbA1c glycated hemoglobin, IGlar insulin glargine (Lantus), LSM least-square mean, LY IGlar Lilly insulin glargine. p > 0.05 for all comparisons between LY IGlar and IGlar in all three populations. p values were < 0.001 for all within-treatment comparisons in all three populations
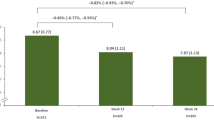
An analysis was done on a subgroup of patients who were on IGlar prior to entering this study. This analysis revealed no statistically significant difference in LSM (standard error) HbA1c change from baseline to Week 24 between LY IGlar and IGlar treatment arms in India (LY IGlar: − 0.85 [0.271]; IGlar: − 0.20 [0.249]; p = 0.092) and East Asia (LY IGlar: − 0.89 [0.153]; IGlar: − 0.99 [0.163]; p = 0.686) subpopulations. This is in agreement with the observations in the ELEMENT 5 total population [13].
The proportion of patients who achieved HbA1c levels < 7.0% and ≤ 6.5% was similar for LY IGlar and IGlar in both the subpopulations in line with the ELEMENT 5 total population. In the India subpopulation, 20.5% (LY IGlar) and 11.4% (IGlar) achieved HbA1c levels < 7.0% and 5.1% (LY IGlar) and 6.8% (IGlar) achieved HbA1c levels ≤ 6.5%. In East Asia subpopulation, 30.3% (LY IGlar) and 43.1% (IGlar) achieved HbA1c levels < 7.0% and 18.2% (LY IGlar) and 16.9% (IGlar) achieved HbA1c levels ≤ 6.5% (Table 2).
A total of 468 out of 493 subjects (95.3%) in the ELEMENT 5 total population, 98 out of 100 subjects (98.0%) in the India subpopulation and 127 out of 134 (94.8%) in the East Asia subpopulation reported FBG data (measurement before morning meal). Least-squares mean [standard error] change in FBG at Week 24 was higher (p = 0.002) for IGlar (− 2.87 [0.10] mmol/L or − 51.8 [1.75] mg/dL) compared with LY IGlar (− 2.41 [0.10] mmol/L or − 43.4 [1.86] mg/dL) in the India subpopulation. Similar observations were recorded for the ELEMENT 5 total population (LY IGlar: − 2.37 [0.08] mmol/L or − 42.8 [1.49] mg/dL; IGlar: − 2.69 [0.08] mmol/L or − 48.4 [1.49] mg/dL; p = 0.007). However, in the East Asia subpopulation, the change in FBG was similar in the two treatment arms (LY IGlar: − 1.9 [0.16] mmol/L or − 34.1 [2.79] mg/dL; IGlar: − 1.99 [0.16] mmol/L or − 35.8 [2.79] mg/dL; p = 0.669; Table 2). The FBG level was similar at all visits for both treatment arms in the East Asia subpopulation. In the India subpopulation, the FBG levels were similar for LY IGlar and IGlar at all visits except at Week 24 (LY IGlar: 5.65 [0.10] mmol/L or 101.8 [1.86] mg/dL; IGlar: 5.18 [0.10] mmol/L or 93.3 [1.75] mg/dL; p = 0.002). This observation in the India subpopulation was similar to the ELEMENT 5 total population (Fig. 2).
Self-monitored fasting blood glucose levels over 24 weeks. a India subpopulation; b East Asia subpopulation; c ELEMENT 5 total population. Values presented are LSM (standard error). Analyses are based on mixed-model repeated-measures. Data points are slightly offset along the x axis to avoid overlapping error bars. FBG fasting blood glucose, IGlar insulin glargine (Lantus), LSM least-squares mean, LY IGlar Lilly insulin glargine. p > 0.05 for all comparisons between LY IGlar and IGlar in all three populations except FBG for ELEMENT 5 total population (p = 0.007) and India (p = 0.002) at Week 24
3.3 Basal Insulin Dose and Body Weight
Basal insulin dose after 24 weeks was similar for LY IGlar and IGlar (Table 2) in both the India and East Asia subpopulations. A significant increase in basal insulin dose from baseline to Week 24 (p < 0.001) was observed for both the treatments. The LSM (standard error) increase observed for LY IGlar was similar to IGlar in both the subpopulations (India—LY IGlar: 16.7 [2.89] U/day, IGlar: 22.4 [2.76] U/day, p = 0.156; East Asia—LY IGlar: 10.0 [2.09] U/day, IGlar: 13.3 [2.09] U/day, p = 0.261).The LSM increase in weight at Week 24 was similar in patients treated with LY IGlar or IGlar for both the subpopulations (Table 2).
3.4 Hypoglycemia
Annualized rates of total hypoglycemia and nocturnal hypoglycemia observed with LY IGlar were similar to those seen with IGlar in both the subpopulations (Table 2). There were no reports of severe hypoglycemia in either subpopulation. There was no statistical difference between the two treatment arms in the proportion of patients who experienced total and nocturnal hypoglycemia during the overall 24-week treatment period for both the subpopulations (Fig. 3).
Incidence of hypoglycemia. a Total hypoglycemia; b Nocturnal hypoglycemia. Data presented are percentage of patients experiencing hypoglycemia measured for the overall 24-week treatment period. IGlar insulin glargine (Lantus), LY IGlar Lilly insulin glargine. p > 0.05 for all comparisons between LY IGlar and IGlar in all three populations
3.5 Adverse Events
Occurrences of AEs including those possibly related to the treatments and injection-site AEs were similar between the two treatments for both the subpopulations, consistent with the observation in the ELEMENT 5 total population. No SAEs were reported in the India subpopulation. The incidence of SAEs in the East Asian population was similar for both treatments. There were no deaths in either of the subpopulations (Table 3).
3.6 Insulin Antibodies
There was no statistically significant difference in the percentage of patients with detectable antibodies between the two treatment arms in both the subpopulations—similar to the ELEMENT 5 total population (Table 2). Median insulin antibody binding percentage with LY IGlar was similar to that observed for IGlar in both the subpopulations. This was consistent with the ELEMENT 5 total population (Table 2, Fig. 4). There was no significant correlation between antibody levels and clinical outcomes as evidenced by the negligible interaction of endpoint insulin antibody levels with HbA1c (India: 0.251; East Asia; 0.522), total hypoglycemia (India: not calculable; East Asia: 0.985), and basal insulin dose (India: 0.9935; East Asia: 0.9729).
Antibody binding levels over 24 weeks. a India subpopulation; b East Asia subpopulation; c ELEMENT 5 total population. Values are presented as median (25th, 75th percentiles). p values for treatment comparisons are derived from the Wilcoxon rank-sum test. Data points are slightly offset along the x axis to avoid overlapping error bars. IGlar insulin glargine (Lantus), LY IGlar Lilly insulin glargine. p > 0.05 for all comparisons between LY IGlar and IGlar in all three populations
3.7 Patient-Reported Outcomes
Patient-reported health outcomes as assessed by the LSM scores of the ITSQ at Week 24 of LY IGlar were comparable with those of IGlar for both India and East Asia subpopulations; this was consistent with the ELEMENT 5 total population (Table 4).
4 Discussion
The ELEMENT 5 study evaluated the efficacy and safety profiles of LY IGlar and IGlar in patients with T2DM. A large proportion of the patients included in ELEMENT 5 study were from India and East Asia. This post hoc analysis of the India and East Asia subpopulations of the ELEMENT 5 study demonstrated that outcomes for the India and East Asia subpopulations were generally similar to those observed for the ELEMENT 5 total population. These results are specifically important since this is the first study comparing LY IGlar and IGlar in Asian patients with T2DM; the only available information for Asian patients so far was from the Japanese subgroup analysis of the ELEMENT 1 study that analyzed LY IGlar and IGlar in patients with type 1 diabetes [20].
The key objective of this analysis, which was to assess if the change in HbA1c from baseline to Week 24 was similar for LY IGlar and IGlar, was achieved in both India and East Asia subpopulations; this observation was consistent with the ELEMENT 5 total population. The change in HbA1c levels from baseline to Week 24 was numerically less in the India subpopulation than that in the East Asia subpopulation and ELEMENT 5 total population. Furthermore, the rate of hypoglycemia in the India subpopulation was also numerically less than that observed in the other populations. Though higher than the India subpopulation, the proportion of patients who achieved HbA1c targets in the East Asia subpopulation was also numerically less than the ELEMENT 5 total population. These findings could be attributed to the fact that there is a more cautious approach to insulin titration in Asia, which may lead to less effective uptitration of the insulin dose resulting in suboptimal glycemic control. It is also possible that fear of hypoglycemia plays a role preventing patients from effective uptitration of insulin dose [21].
Several other studies have also reported similar findings in Asian versus non-Asian patients with T2DM. A pooled analysis of 16 randomized controlled trials involving Asian and non-Asian T2DM patients receiving insulin glargine reported that fewer Asians achieved the target HbA1c levels [22]. The PARADIGM post hoc analysis [23] and the EDGE study [24] have also reported similar findings.
In the ELEMENT 5 study, patients used a self-titration algorithm through which they increased their insulin dose by 1 U/day until FBG levels were lower than 5.6 mmol/L (100 mg/dL). The finding that FBG levels at Week 24 were very close to the set target demonstrated success of the self-titration program. We also observed that the change in FBG levels was higher for IGlar as compared with LY IGlar in the India subpopulation at Week 24, while it was similar at all other time points. There were no similar findings in other subgroups. Furthermore, incidence of hypoglycemia including nocturnal hypoglycemia was similar between the two treatment arms. Based on these observations, we considered the difference in change in FBG levels to be a chance finding without any medical significance.
Both LY IGlar and IGlar were well tolerated in the India and East Asia subpopulations. Safety profiles were similar between the treatment arms for both subpopulations and were consistent with the ELEMENT 5 total population. In fact, fewer patients from the two subpopulations experienced treatment-emergent AEs than the total population. In the India subpopulation, a numerically lower proportion of patients treated with LY IGlar experienced treatment-emergent AEs as compared with IGlar.
There are several limitations to the ELEMENT 5 study and the subpopulation analyses. The ELEMENT 5 study was not particularly designed to study the ethnic differences and region-specific factors influencing diabetes prevalence. In addition, the study was not blinded due to the distinct appearances of the two insulin glargine interventions. This might have influenced the actions of patients and investigators. Using the double-dummy technique could have blinded the distinct appearances of the two pen devices. However, injecting these would have imposed substantial burden on the patients. Furthermore, the subpopulation analyses were done post hoc and the size of both subpopulations was small. These analyses were not powered to make statistical comparisons between the two subpopulations or with the ELEMENT 5 total population. No adjustments were made to the p values to adjust for multiplicity. However, the numbers were similar in all the subgroups; thereby ensuring uniformity.
5 Conclusion
The subpopulation analyses in the Indian and East Asian patients of the ELEMENT 5 study showed that LY IGlar and IGlar, when administered in combination with OAMs, have similar efficacy and safety profiles. These observations were consistent with the ELEMENT 5 total population.
References
IDF Diabetes Atlas Eighth edition. 2017. Available from: http://diabetesatlas.org/resources/2017-atlas.html. Accessed July 2018.
Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32(1):193–203.
Davies MJ, D’Alessio DA, Fradkin J, et al. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669–701.
Owens DR. Insulin preparations with prolonged effect. Diabetes Technol Ther. 2011;13(Suppl 1):S5–14.
Hilgenfeld R, Seipke G, Berchtold H, Owens DR. The evolution of insulin glargine and its continuing contribution to diabetes care. Drugs. 2014;74(8):911–27.
Heinemann L, Linkeschova R, Rave K, Hompesch B, Sedlak M, Heise T. Time-action profile of the long-acting insulin analog insulin glargine (HOE901) in comparison with those of NPH insulin and placebo. Diabetes Care. 2000;23(5):644–9.
Riddle MC, Rosenstock J, Gerich J, Insulin Glargine 4002 Study Investigators. The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26(11):3080–6.
Lepore M, Pampanelli S, Fanelli C, et al. Pharmacokinetics and pharmacodynamics of subcutaneous injection of long-acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes. 2000;49(12):2142–8.
Davies M, Dahl D, Heise T, Kiljanski J, Mathieu C. Introduction of biosimilar insulins in Europe. Diabet Med. 2017;34(10):1340–53.
Tieu C, Lucas EJ, DePaola M, Rosman L, Alexander GC. Efficacy and safety of biosimilar insulins compared to their reference products: A systematic review. PLoS One. 2018;13(4):e0195012.
Ilag LL, Costigan TM, Deeg MA, et al. Clinical outcomes of patients with diabetes who exhibit upper-quartile insulin antibody responses after treatment with LY2963016 or Lantus® Insulin Glargine. Diabetes Ther. 2017;8(3):545–54.
A study of LY2963016 compared to LANTUS® in adult participants with type 2 diabetes mellitus (ELEMENT 5). https://clinicaltrials.gov/ct2/show/results/NCT02302716. Accessed Jan 2019.
Pollom RK, Ilag LL, Lacaya LB, Morwick TM, Ortiz Carrasquillo R. Lilly insulin glargine versus Lantus® in insulin-naive and insulin-treated adults with type 2 diabetes: a randomized, controlled trial (ELEMENT 5). Diabetes Ther. 2019;10(1):189–203.
Rhee EJ. Diabetes in Asians. Endocrinol Metab (Seoul). 2015;30(3):263–9.
Unnikrishnan R, Anjana RM, Mohan V. Diabetes mellitus and its complications in India. Nat Rev Endocrinol. 2016;12(6):357–70.
Tsai ST, Pathan F, Ji L, et al. First insulinization with basal insulin in patients with Type 2 diabetes in a real-world setting in Asia. J Diabetes. 2011;3(3):208–16.
Mohan V, Shah SN, Joshi SR, et al. Current status of management, control, complications and psychosocial aspects of patients with diabetes in India: results from the DiabCare India 2011 Study. Indian J Endocrinol Metab. 2014;18(3):370–8.
Hu FB. Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care. 2011;34(6):1249–57.
Anderson RT, Skovlund SE, Marrero D, et al. Development and validation of the insulin treatment satisfaction questionnaire. Clin Ther. 2004;26(4):565–78.
Nishiyama H, Shingaki T, Suzuki Y, Ilag LL. Similar intrapatient blood glucose variability with LY2963016 and Lantus® Insulin Glargine in patients with type 1 (T1D) or type 2 diabetes, including a Japanese T1D subpopulation. Diabetes Ther. 2018;9(4):1469–76.
Chan WB, Chen JF, Goh SY, et al. Challenges and unmet needs in basal insulin therapy: lessons from the Asian experience. Diabetes Metab Syndr Obes. 2017;10:521–32.
Chan JCN, Bunnag P, Chan SP, et al. Glycaemic responses in Asian and non-Asian people with type 2 diabetes initiating insulin glargine 100 units/mL: a patient-level pooled analysis of 16 randomised controlled trials. Diabetes Res Clin Pract. 2018;135:199–205.
Ji L, Min KW, Oliveira J, Lew T, Duan R. Comparison of efficacy and safety of two starting insulin regimens in non-Asian, Asian Indian, and East Asian patients with type 2 diabetes: a post hoc analysis of the PARADIGM study. Diabetes Metab Syndr Obes. 2016;9:243–9.
Brath H, Paldanius PM, Bader G, Kolaczynski WM, Nilsson PM. Differences in glycemic control across world regions: a post-hoc analysis in patients with type 2 diabetes mellitus on dual antidiabetes drug therapy. Nutr Diabetes. 2016;6(7):e217.
Acknowledgements
We thank the participants of the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Eli Lilly (Indianapolis, IN, USA) and Boehringer Ingelheim (Ingelheim am Rhein, Germany). Eli Lilly and Company funded the open-access fee. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Authorship
All named authors meet the International Committee of Medical Journal Editors criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Medical writing and editorial assistance
The authors acknowledge Rajneeta Roy, an employee of Eli Lilly Scientific Services Private Limited for medical writing and editorial assistance.
Conflict of interest
Viswanathan Mohan and Manoj Chadha have received speaker fees from Eli Lilly and Company, Novo Nordisk and Sanofi. Young Min Cho has received research support or consultant fees from Astrazeneca, Sanofi, LG chemicals, and Hanmi. Sanjay Kalra has received speaker fees from Eli Lilly and Company, Novo Nordisk, and Sanofi. Rakesh Kumar Sahay has received speaker fees from Eli Lilly and Company, Novo Nordisk, and USV India. Indranil Bhattacharya, So Yeon Kim, and Erik Spaepen are employees of Eli Lilly and Company. Chien-Ning Huang, and Kyu Jeung Ahn do not have any conflict of interest.
Ethical approval
The study was conducted in accordance with International Council for Harmonization Guidelines for Good Clinical Practice and the Declaration of Helsinki following approvals by the pertinent ethics boards. All patients provided written informed consent prior to entering the study.
Data availability
The data sets analyzed during the current study are not publicly available. Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available on request 6 months after the indication studied has been approved in the USA and EU and after primary publication acceptance, whichever comes later. No expiration date of data requests is currently set once they are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment for up to 2 years per proposal. For details on submitting a request, see the instructions provided at http://www.clinicalstudydatarequest.com.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Mohan, V., Ahn, K.J., Cho, Y.M. et al. Lilly Insulin Glargine Versus Lantus® in Type 2 Diabetes Mellitus Patients: India and East Asia Subpopulation Analyses of the ELEMENT 5 Study. Clin Drug Investig 39, 745–756 (2019). https://doi.org/10.1007/s40261-019-00798-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-019-00798-1