Abstract
Intravenous gadobutrol [Gadovist™ (EU); Gadavist® (USA)] is a second-generation, extracellular non-ionic macrocyclic gadolinium-based contrast agent (GBCA) that is approved for use in paediatric (including term neonates) and adult patients undergoing diagnostic contrast-enhanced (CE) MRI for visualization of pathological lesions in all body regions or for CE MRA to evaluate perfusion and flow-related abnormalities. Its unique physicochemical profile, including its high thermostability and proton relaxation times, means that gadobutrol is formulated at twice the gadolinium ion concentration of other GBCAs, resulting in a narrower bolus and consequently, improved dynamic image enhancement. Based on > 20 years of experience in the clinical trial and real-world settings (> 50 million doses) and its low risk for developing nephrogenic systemic fibrosis (NSF), gadobutrol represents an effective and safe diagnostic GBCA for use in CE MRI and MRA to visualize pathological lesions and vascular perfusion and flow-related abnormalities in all body regions in a broad spectrum of patients, including term neonates and other paediatric patients, young and elderly adult patients, and those with moderate or severe renal or hepatic impairment or cardiovascular (CV) disease.
Similar content being viewed by others
Extensive clinical experience confirms its diagnostic efficacy in CE MRI of pathological lesions in all body regions in a broad spectrum of patients |
Strong agreement for the diagnosis of flow-related abnormalities between gadobutrol-enhanced MRA and intra-arterial digital subtraction angiography |
Very good safety profile |
As a macrocyclic GBCA, it poses a low risk for the development of NSF, with no robust evidence for an increase in signal intensity in CNS CE-MRI after multiple administrations (unlike linear GBCAs) |
1 Introduction
More than two decades of clinical experience has firmly established the diagnostic efficacy and safety of gadolinium-based contrast agents (GBCAs) in contrast-enhanced (CE) MRI to detect pathological lesions throughout the body and in CE MRA to detect peripheral arterial occlusive disease (PAOD) or flow-related abnormalities [1]. As high concentrations of free gadolinium (Gd3+) ions are potentially toxic, all GBCAs are formulated as a central Gd3+ ion surrounded by a linear or macrocyclic chelate ligand [1,2,3,4].
This article reviews the extensive clinical experience (> 20 years) for the use of intravenous gadobutrol [Gadovist™ (EU); Gadavist® (USA)] as a second-generation, extracellular macrocyclic contrast medium in patients undergoing diagnostic CE MRI or CE MRA, with a brief overview of the pharmacological properties of gadobutrol. Some of these data have been previously reviewed in Clinical Drug Investigation [5].
2 Physicochemical Properties of Gadobutrol
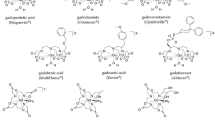
Gadobutrol is a highly water soluble, hydrophilic GBCA that is bound to a non-ionic, macrocyclic rigid chelate complex of high kinetic (i.e. exhibits slow kinetics of decomplexation) and thermodynamic stability (Table 1) [5,6,7]. Its unique physiochemical profile means that gadobutrol is formulated at twice the concentration of Gd3+ ions as other GBCAs (1.0 vs. 0.5 mol/L solution) [6, 8]. The higher concentration of gadobutrol reduces its injection volume by 50%, providing a narrower bolus and thereby, improving dynamic image enhancement [9, 10]. Gadobutrol exhibits the greatest shortening of proton T1 relaxation times amongst all macrocyclic GBCAs [7]. Shortening of proton relaxation time is a key determinant of signal and contrast enhancement in MRI [6].
3 Pharmacokinetic Properties of Gadobutrol
Intravenous gadobutrol exhibits dose-proportional pharmacokinetics, is rapidly distributed into the extracellular space and shows minimal plasma protein binding [11, 12]. Gadobutrol is primarily eliminated in the urine, with > 50, > 90 and 100% of the dose eliminated in the urine within 2, 12 and 72 h, respectively [11, 12]. The mean elimination half-life of gadobutrol is 1.8 h, which corresponds to the renal elimination rate in healthy individuals [11, 12]. Gadobutrol is not metabolized [11], with no metabolites detected in the plasma or urine [12].
Overall, the pharmacokinetic profile of gadobutrol in paediatric patients of all ages, including infants aged < 2 years (range < 1 to 23 months; n = 43) [13] and children aged 2–17 years (n = 130) [14], was similar to that in adults [11, 12]. No dosage adjustments are required in paediatric patients, including term neonates [11, 12]. Based on data from children aged 2–17 years, most of an administered dose was recovered in the urine within 6 h (median recovery ≈ 99%) [11, 12]. There was also no clinically relevant difference in the pharmacokinetic profile of gadobutrol in healthy volunteers aged ≥ 65 years versus that in younger adults [11, 12].
Since gadobutrol is primarily eliminated renally, the serum half-life of gadobutrol is prolonged in patients with impaired renal function and correlates with the reduction in creatinine clearance [11, 12, 15]. Within 72 h post injection, there was complete recovery of gadobutrol in the urine in patients with mild to moderate renal impairment; in those with severe renal impairment, ≈ 80% of the dose was recovered within 5 days [11, 12, 15]. In patients with renal impairment who required haemodialysis, 98% of gadobutrol was removed after three haemodialysis sessions [11, 12, 16].
4 Diagnostic Efficacy of Gadobutrol
Discussion in this section focuses on large (n > 100), multicentre, phase 3 or 4 trials, where available. Exclusion criteria in these trials included general contraindications to MRI or MRA (e.g. previous GBCA hypersensitivity; presence of an implanted metallic device), clinical instability or interventions changing the findings in target tissue or vessels. All GBCAs were administered intravenously, with the dose based on bodyweight. Unless stated otherwise, the dose of each GBCA was 0.1 mmol/L/kg. Quantitative and qualitative diagnostic efficacy assessments were made in a blinded manner by ≤ 3 independent, off-site radiologists.
Clinical trials evaluating the diagnostic efficacy of gadobutrol in paediatric patients (including term neonates) are more limited. Approval of gadobutrol for CE MRI in these patients was based on prospective, multinational, pharmacokinetic (Sect. 3) and safety (Sect. 5) studies in children aged 2–17 years [14] and infants aged ≤ 2 years (mean age 8.8 months; range 0.2–23 months) [13], and on clinical experience in adults [11]. Diagnostic efficacy and an increase in diagnostic confidence was shown for all parameters assessed in children aged 2–17 years having CE MRI of the CNS, liver and kidneys, or CE MRA [12, 14] and in infants aged ≤ 2 years scheduled for CE MRI of any body region [12, 13]. There was no difference in diagnostic efficacy results among paediatric age groups or between paediatric and adult patients [12]. These data are supported by a subgroup analysis of 1142 children (aged < 18 years) [17] enrolled in the prospective, noninterventional, multinational GARDIAN study (n > 23,000) [18], with investigators rating gadobutrol-enhanced MRI as good or excellent in 98% of paediatric patients [17]. Further support for its diagnostic efficacy in very young paediatric patients comes from a single-centre, observational study in 60 infants aged 4 days to 22.7 months (mean age 11.1 months) who underwent gadobutrol-enhanced MRI of various body regions or MRA [19].
4.1 MRI of CNS
4.1.1 In Patients with Malignant CNS Lesions
Pivotal phase 3 [20,21,22,23,24] and 4 [25, 26] trials have firmly established the diagnostic efficacy of gadobutrol in adult patients undergoing diagnostic CE MRI of brain lesions (Table 2), including for diagnosing primary and metastatic brain tumours. With the exception of the REMIND trial in patients with primary brain tumours [25] and a phase 3 trial in patients with known or suspected brain metastases [24], all other trials included patients with primary or metastatic brain tumours [20,21,22,23, 26] and, in some instances, also included patients with non-tumour brain lesions (e.g. white matter disease, vascular lesions, infarct, haemorrhage, infective/inflammatory disease) [20, 21, 26].
In phase 3 trials, the diagnostic efficacy of combined gadobutrol-enhanced plus unenhanced MRI was noninferior to unenhanced MRI for the mean number of lesions detected/patient (primary quantitative outcome) and superior to unenhanced MRI for qualitative primary outcomes of lesion contrast enhancement (LCE), border delineation (LBD) and internal morphology (LIM) (Table 2) [20, 21, 23]. There was no loss of specificity, exact-match diagnostic accuracy or reader confidence associated with gadobutrol-enhanced improvements in the sensitivity and accuracy of malignant lesion detection [21, 23].
The overall preference for LCE significantly (p > 0.001) favoured gadobutrol over gadoterate meglumine MRI in a single-blind, phase 3 trial (Table 2) (primary outcome) [22]. In REMIND [25], gadobutrol was noninferior to gadoterate meglumine for overall lesion visualization and characterization (primary outcome), with a high percentage (> 90%) of lesion images rated as good or excellent for these parameters with both GBCAs (Table 2). Readers also had no specific preference for either GBCA for secondary qualitative outcomes, including diagnostic confidence (Table 2) [25]. Quantitative outcomes in these two trials also generally favoured gadobutrol over gadoterate meglumine MRI (Table 2) [22, 25].
Gadobutrol generally exhibited similar diagnostic efficacy to gadoteridol for primary qualitative outcomes, including LCE, LBD and/or LIM, in phase 3 trials [20, 24] and the phase 4 TRUTH trial [26] (Table 2). Where evaluated, gadobutrol 0.1 and 0.2 mmol/L/kg were noninferior to gadoteridol 0.2 mmol/L/kg for these outcomes [20]. Gadobutrol 0.1 and 0.2 mmol/L/kg were also noninferior to gadoteridol 0.2 mmol/L/kg for the mean number of detected lesions/patient (primary quantitative outcome) in one phase 3 trial [24], although noninferiority was not demonstrated in the other [20] (Table 2). In TRUTH, readers had no specific preference for either GBCA for other qualitative outcomes, including global diagnostic preference or disease extent outcomes, or for quantitative assessments (Table 2) [26].
4.1.2 In Patients with Multiple Sclerosis
Gadobutrol-enhanced MRI was effective for imaging acute inflammatory multiple sclerosis (MS) lesions in a randomized, intra-individual crossover, multicentre trial in adults with known or suspected active MS lesions (n = 45 evaluable) [27]. There were no significant differences between gadobutrol and gadoterate meglumine MRI for qualitative preference ratings for LCE (primary outcome), LBD or overall preference scores. The vast majority of gadobutrol and gadoterate meglumine images for all assessed timepoints were rated as excellent by both readers. There was no difference between these two GBCAs for the median number of enhancing lesions detected in each post-contrast image (median of 2 lesions with each GBCA). At 3, 6 and 9 mins post-contrast agent, gadobutrol generated significantly (p < 0.05) higher signal intensity than gadoterate meglumine [27].
4.2 MRA
4.2.1 Peripheral Arteries
The diagnostic efficacy of gadobutrol-enhanced MRA compared with that of intra-arterial digital subtraction angiography (DSA; gold standard) was established in pivotal phase 3 [28, 29] or 4 [30] trials in patients with PAOD [28, 30] or various arterial occlusive diseases (e.g. pelvic artery disease, cerebral ischaemia, thoracic or abdominal aortic aneurysms, renal artery stenosis) [29] (Table 3). Overall, gadobutrol-enhanced MRA provided accurate, rapid and non-invasive evaluation of peripheral arteries in these patient populations [28,29,30].
In patients with PAOD, there was strong agreement between gadobutrol-enhanced MRA and DSA results for accuracy for diagnosing occlusion of body arteries (primary outcome) (Table 3) [28, 30]. Relative to DSA, gadobutrol-enhanced MRA was noninferior to gadoterate meglumine-enhanced MRA for diagnostic accuracy (primary outcome), with both GBCAs showing similar efficacy for sensitivity and specificity for detecting significant stenosis (i.e. > 50% stenosis) (Table 3) [30]. There were also no significant differences between gadobutrol and gadoterate meglumine for diagnostic confidence (86 vs. 87%), CNR (155.3 vs. 159.5) or signal-to-noise ratio (SNR) (161.0 vs. 165.5) [30].
There was also strong agreement in diagnostic efficacy between gadobutrol-enhanced MRA and DSA in patients with various arterial occlusive diseases for both off-site (Table 3) and on-site reader results, based on whole-body MRA [29]. Overall, sensitivities and specificities for detection of relevant stenosis appeared to be similar between gadobutrol-enhanced MRA and DSA (Table 3), as were positive predictive values (PPV) and negative predictive values (NPV) for the detection of relevant stenosis. Respective on-site and off-site PPV for the internal carotid artery, common iliac artery and external iliac artery were 79–96 and 53–91.5%, with NPV for on-site and off-site evaluations of 98–99 and 84–99% [29].
4.2.2 Cerebral Vessels
Gadobutrol-enhanced MRA showed diagnostic efficacy for visualization of cerebral vessels in prospective studies in adult patients with cerebrovascular [31] or vascular [29] disease, with the latter trial discussed in Sect. 4.2.1 [29]. Gadobutrol-enhanced MRA was associated with significantly (p = 0.041) better delineation of intracranial vessels than gadoterate meglumine-enhanced MRA in an analysis of 1026 cervicocranial vessel segments from 54 patients, based on intra-individual comparisons of the two GBCAs [31]. This improved delineation reflects the significantly higher CNR (mean 178.7 vs. 162.3; p = 0.031) and SNR (208.3 vs. 191.1; p = 0.032) values with gadobutrol than gadoterate meglumine across all vessel segments. Typically, SNR and CNR values were also significantly (p < 0.05 vs. gadoterate meglumine) higher with gadobutrol for thoracic, cervical and intracranial vessels. Based on the consensus read, the overall preference for an individual contrast agent was markedly higher for gadobutrol than gadoterate meglumine images (40.7 vs. 16.7%; p = 0.02), with the GBCAs rated as equal in 42.6% of cases [31]. This trial is supported by evidence from a randomized study in 20 healthy adult volunteers [10].
4.3 MRI of Other Body Regions
4.3.1 Kidney and Liver
The diagnostic efficacy of gadobutrol for CE MRI for known or suspected liver [32] or renal [33] lesions was established in pivotal, multinational, parallel-group phase 3 trials (Table 4). In these trials, the diagnostic accuracy of gadobutrol was noninferior to that of gadopentetate dimeglumine for the classification of liver or kidney lesions, with respective diagnostic accuracy rates for each contrast medium of ≥ 80 and ≥ 84% (primary outcome) (Table 4). In general, similar rates for diagnostic sensitivity and specificity for pre- versus post-contrast MRI were observed with both GBCAs (Table 4), with the increase in these rates from unenhanced MRI generally similar for both GBCAs [32, 33].
4.3.2 Breast
Two large (n > 385 evaluable/trial), multinational phase 3 trials (GEMMA1 and GEMMA2) confirmed the diagnostic efficacy of gadobutrol-enhanced preoperative breast MRI for detecting malignant breast lesions (Table 4) [34]. For the coprimary outcomes, gadobutrol-enhanced breast MRI was superior to unenhanced breast MRI for intra-patient MRI sensitivity (i.e. breast-level sensitivity) and, based on cancer-free breasts as reference standard, provided superior breast-level specificity for correctly excluding malignancy in cancer-free breasts (Table 4). In malignant breasts, gadobutrol-enhanced MRI breast-level sensitivities ranged from 47 to 61% across the six readers in GEMMA1 and GEMMA2 and respective median NPV in these two studies were 96 and 94% [34].
These data are supported by small (n > 50), prospective, single-blind, single-centre [35] or multicentre [36], crossover studies in women with biopsy-proven [36] or suspected [35] breast cancer. In the multicentre study (n = 72), there was a 94% agreement rate for detection of the index lesion between gadobutrol-enhanced and gadobenate dimeglumine-enhanced MRI, with no significant difference between the contrast agents for sensitivity in lesion detection of all detected lesions (82.33 vs. 81.6%) [36]. In the other study (n = 52), gadobutrol-enhanced MRI was noninferior to gadobenate meglumine-enhanced MRI for breast lesion detection and sensitivity in lesion characterization [35]. A retrospective study of 400 patients with histologically-confirmed breast cancer provides further support for the diagnostic efficacy of gadobutrol-enhanced MRI in detecting breast lesions [37].
The ongoing, observational, multicentre MIPA study enrolled two concurrent groups of patients with newly-diagnosed breast cancer, not candidate to neoadjuvant therapy, receiving or not receiving MRI (abstracts) [38, 39]. As of July 2016, data from 2425 patients were available for analysis, 1224 of whom (50.5% patients) had received preoperative breast CE MRI (gadobutrol was used in 70% of these CE MRI). Of the 1224 CE MRIs, 17% were performed for screening or diagnosis purposes, with CE MRI typically used as a confirmation tool for an already planned mastectomy. CE MRI was not associated with an increased rate of mastectomy (increased by 1.7% vs. the non-MRI group) [38, 39].
The sensitivity of mammographic detection of tumour lesions is significantly reduced in women with extremely dense breasts, with these women having an increased risk of breast cancer [40]. The ongoing randomized, parallel-group DENSE trial, which is being conducted in a Dutch breast cancer screening cohort with extremely dense breasts, is investigating the value of adding gadobutrol-enhanced MRI to routine 2-year breast mammography screening (n = 7237 planned enrolments) compared with routine mammography alone (n = 28,948) [40]. The primary aim is to detect a statistically significant reduction in the interval cancer rate of the intervention arm (i.e. mammography plus MRI) versus routine mammography alone [40].
4.3.3 In Other Body Regions
A large (n = 346 per-protocol set), single-blind, multinational phase 3 trial investigated the diagnostic efficacy of gadobutrol-enhanced MRI in patients with various underlying pathologies, including neurological, vascular, liver, kidney, breast, cardiovascular (CV) and musculoskeletal disorders [41]. Gadobutrol was noninferior to gadopentetate dimeglumine for CE MRI of various body regions and the extremities, based on the average reader total score for the three visualization variables (i.e. LCE + LBD + LIM; mean total score 9.39 vs. 9.35) [primary outcome]. Both GBCAs showed high sensitivity (≥ 82%) and specificity (≥ 78%) for detecting malignant lesions (Table 4) [41].
Small (n = 12–30), prospective, single-centre studies in patients with chronic myocardial infarction (MI) [42,43,44,45] or hypertrophic cardiomyopathy [46] investigated the diagnostic efficacy of gadobutrol-enhancement in CV MRI. Single doses of gadobutrol or gadobenate dimeglumine were effective for late gadolinium-enhancement (LGE) imaging of chronic MI, based on intra-individual comparisons [44]. There were no significant differences between these two GBCAs for the mean SNR for infarct, remote myocardium or ventricular blood. There was also no significant difference for the mean CNR between infarct and myocardium, although gadobutrol enhancement resulted in a higher CNR between the infarct and blood than gadobenate dimeglumine enhancement (4.0 vs. 0.9; p = 0.02) [44]. Compared with gadopentetate dimeglumine, gadobutrol 0.15 mmol/kg provided similar delineation of the infarct scar in another study, based on SNR values between scar tissue and blood, and CNR values between scar tissue and remote myocardium or blood [42]. Except for the CNR between scar tissue and blood, these SNR and CNR values were significantly (p ≤ 0.0001) lower with gadobutrol 0.10 mmol/kg than those for gadopentetate dimeglumine (i.e. reduced tissue contrast with this dose of gadobutrol) [42]. In another study in 20 patients, there were no statistically significant differences between gadobutrol and gadopentetate dimeglumine for detection of late enhancement in cardiac MRI [45].
Preliminary retrospective studies in patients with prostate cancer (n = 34 [47] and 53 [48]) suggested that gadobutrol was effective for dynamic CE MRI detection of prostate lesions. In the largest study, gadobutrol was associated with significantly (p = 0.04) higher peak enhancement in prostate cancer lesions and in the normal peripheral zone around the lesion than gadopentetate dimeglumine [48]. There were no significant differences between these GBCAs for curve type frequencies (i.e. type I, II and III curves) [48].
The sensitivity of gadobutrol-enhanced MRI for detecting small, (i.e. < 3 cm) solid pancreatic lesions (SPLs) was significantly higher than that with CT imaging in 193 patients with SPLs (98–99.5 vs. 91–93%; p < 0.05) [49]. There was no significant difference between the two imaging techniques for the specificity of detecting SPL (100% for both techniques for both readers). Overall, SPL were significantly (p < 0.001) more conspicuous on gadobutrol-enhanced MRI than CT images [49].
5 Tolerability and Safety of Gadobutrol
As a contrast agent for MRI and MRA, intravenous gadobutrol has a very good and well established safety profile based on extensive evidence (> 50 million doses as of April 2018 [50]) from the clinical trial and real-world settings [8, 17, 18, 51,52,53,54,55,56], with most adverse drug reactions (ADRs) of mild to moderate intensity and transient in nature [8, 11, 12, 18]. The most common ADRs (incidence 1–10%) were headache and nausea [12]. In global postmarketing surveillance reports (> 29.6 million gadobutrol doses), ADRs were uncommon (incidence 0.05%), with anaphylactoid/hypersensitivity reactions (reporting rate 0.019%), nausea/retching (0.005%) and vomiting (0.004%) the most common ADRs reported [52]. Most ADRs were of mild (83.6%) or moderate (13.2%) intensity, transient and occurred within 5 min (62%) or within the next 24 h after gadobutrol injection. ADRs were not dose related, with incidences of 0.61, 0.78, 0.83 and 0% in the gadobutrol ≤ 1, > 0.1–0.2, > 0.2–0.3 and > 0.3 mmol/L/kg groups, respectively [18]. The very low rates of acute ADRs (pooled analysis of six studies) [51] and allergic ADRs [57] after gadobutrol administration were confirmed in several large prospective observational studies.
The tolerability and safety profile of gadobutrol was similar across all age groups [8, 18, 51,52,53], including in term neonates [13, 19], paediatric patients (aged > 18 years) [8, 13, 17, 18, 51,52,53] and elderly patients (aged ≥ 60 [51] or ≥ 65 [18, 52, 54] years). Evidence from prospective multinational postmarketing surveillance studies (GARDIAN [18] and GRIP [58]) and large pooled analyses [8, 52, 53] also indicated that the safety profiles of gadobutrol in patients with renal or hepatic impairment or with cardiac disease were similar to that in the general population. For example, in the prospective GARDIAN study conducted in the routine clinical practice setting (n > 23,000), ADRs occurred with a frequency of 0.7% in the overall population (n = 23,708), 0% of renally impaired patients (n = 153), 0.9% of patients with cardiac disease (n = 1233) and 0.5% of paediatric patients (n = 1142) having gadobutrol-enhanced MRI or MRA [18]. All ADRs in patients with cardiac disease were of a non-cardiac nature [18]. In pooled safety analyses [53, 55, 56], there were no clinically relevant effects of gadobutrol on any of the ECG parameters or vital signs, including in patients with CV disease. Gadobutrol 0.1–0.5 mmol/L/kg had no clinically relevant influence on heart rate, cardiac rhythm, pacing disturbances (extra systoles), cardiac conduction or QT intervals in healthy adult volunteers, based on a randomized, double-blind, crossover, thorough QT study [59].
The safety profile of gadobutrol was similar to those of other GBCAs, based on pooled safety analyses of clinical trials and postmarketing surveillance data from patients having gadobutrol-enhanced MRI and MRA [8, 52, 53] and a retrospective analysis (n = 10,608 MRIs) [60]. For example, in the most recent pooled analysis of 42 phase 2–4 clinical trials, 3.5% of patients in both the gadobutrol (n = 6809, including 182 children) and GBCA (n = 2184 adults) groups experienced ADRs, most commonly nausea (incidence 0.7% in both groups) [52]. In the gadobutrol and other GBCA groups, rates of serious adverse events (< 0.1 vs. 0%) and hypersensitivity reactions (< 0.1 vs. 0%) were low [52].
5.1 Nephrogenic Systemic Fibrosis
Like other macrocyclic GBCAs, gadobutrol has a low propensity to be associated with nephrogenic systemic fibrosis (NSF) [53, 61]. After > 32 million applications of gadobutrol in routine clinical practice through to 2016, 13 cases of NSF or NSF-like symptoms were reported, with 5 of these 13 patients receiving gadobutrol only (i.e. no other GBCA) [62]. Of the five patients receiving gadobutrol only, three patients met the criteria for being diagnostic of or consistent with NSF, and a possible association with gadobutrol could not be excluded [62], based on a conservative worst-case scenario criteria (as described by Girardi et al. [63]). Interpretation of the other eight cases was confounded by the use of multiple GBCAs, including gadobutrol [62]. Furthermore, after > 50 million doses of gadobutrol, no further cases diagnostic of or consistent with NSF have been reported [50]. In GARDIAN, no paediatric or adult patients experienced NSF-related signs or symptoms [17, 18]. In the GRIP study in patients with mild (eGFR > 65 mL/min/1.73 m2 at baseline; n = 38; not included in follow-up analysis), moderate (eGFR ≥ 30 to ≤ 59 mL/min/1.73 m2 at baseline; n = 540) or severe (eGFR < 30 mL/min/1.73 m2 at baseline; n = 201) renal impairment, no patients with moderate or severe renal impairment developed symptoms indicative of NSF during the 24-month follow-up period after gadobutrol administration (primary outcome) [58].
GBCAs may increase the risk of NSF among patients with impaired elimination of these drugs [12]. The risk of NSF is highest amongst patients with chronic severe kidney disease [glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2] or acute kidney injury [11, 12]. Patients undergoing liver transplantation are at particular risk, since the incidence of acute renal failure is high in this patient population [12]. The use of GBCAs should be avoided amongst at-risk patients unless the diagnostic information is essential and is not available with non-contrast MRI or other modalities [11, 12].
6 Dosage and Administration of Gadobutrol
Intravenous gadobutrol 1 mol/L solution is indicated in numerous countries globally, including EU countries [12], the USA [11] and Japan [64], for use as a contrast agent for diagnostic MRI and/or MRA. Specific indications may differ between countries. In the EU [12], gadobutrol is indicated in adults and children of all ages (including term neonates) for contrast enhancement in cranial and spinal MRI, contrast enhanced MRI of liver or kidneys in patients with high suspicion or evidence of having focal lesions to classify these lesions as benign or malignant and for CE MRI of pathologies of the whole body, and for CE in MRA. In the USA [11], gadobutrol is indicated in adults and children of all ages (including term neonates) for contrast enhancement to detect and visualize areas with disrupted blood brain barrier and/or abnormal vascularity of the CNS, to assess the presence or extent of malignant breast disease in adults and to evaluate known or suspected supra-aortic or renal artery disease in adults and children of all ages (including term neonates).
Gadobutrol is given as a single bolus intravenous infusion; CE MRI may commence immediately afterwards [11, 12]. The dose should be calculated based on a patient’s bodyweight, with a recommended dose for CE MRI in adults and paediatric patients of 0.1 mmol/L/kg. For CE MRA, a fixed volume is recommended based on bodyweight and the number of fields of view [11, 12]. Local prescribing information should be consulted for detailed information, including specific indications, precautions, contraindications and use in special patient populations.
7 Place of Gadobutrol in CE MRI and MRA
The crucial role of CE MRI and MRA in diagnostic and follow-up imaging of pathological lesions is widely recognized across almost all fields of medicine, with these imaging techniques the preferred choice for many conditions, including neurological conditions, MS, cancers involving soft tissues, and abnormalities in vascular anatomy throughout the body [1, 65, 66]. Indeed, CE MRI and MRA offer potential advantages over and are often used as an alternative to ultrasonography and CT for the evaluation of acute spinal cord injury, ischaemic heart disease, complex congenital heart disease and abnormalities in vascular flow throughout the body [1, 66, 67]. CE MRI and MRA are less invasive techniques than CT, provide improved reproducibility and a lower propensity for inter-reader variability than ultrasonography, and are associated with increased soft tissue resolution and differentiation (especially for smaller lesions) versus CT imaging, without the risk of exposure to ionizing radiation [1, 66, 67].
Extensive clinical experience in the clinical trial and/or real-world settings in a broad spectrum of patients (including term neonates, paediatric and adult patients, and those with moderate to severe renal or hepatic impairment of CV disease) has firmly established the very good diagnostic efficacy of gadobutrol-enhanced MRI for visualizing pathological lesions in all body regions, including in the CNS (e.g. tumours, MS) (Sect. 4.1), kidney (Sect. 4.3.1), liver (Sect. 4.3.1), breast (Sect. 4.3.2) and other body regions (e.g. heart, prostate, pancreas) (Sect. 4.3.3). In addition, in patients with PAOD or other flow-related abnormalities, CE MRA provided very effective visualization of peripheral and CNS flow abnormalities (Sect. 4.2). Indeed, in pivotal trials, there was significant agreement in the diagnosis of flow-related abnormalities in peripheral vessels between CE MRA and DSA (gold standard imaging technique) (Sect. 4.2.1).
The stability of Gd3+ ions in GBCAs is clinically relevant, since the release of Gd3+ ions from the chelate ligand into the body may be associated with the development of NSF in patients with severe renal impairment [2, 68]. In view of its high stability and low propensity to release Gd3+ ions, gadobutrol was placed in the lowest risk category for development of NSF amongst GBCAs [1, 3, 4, 69].
More recently, increased signal intensity on unenhanced T1 W MRI and the presence of gadolinium in the brain of patients with normal renal function have been reported [70, 71], with increased signal intensity robustly associated with linear rather than macrocyclic GBCAs such as gadobutrol [72,73,74,75,76,77]. Trace amounts of gadolinium have also been measured at autopsy in the brain and other body regions after both linear and macrocyclic GBCAs [78,79,80,81,82,83,84]. To date, none of the many imaging studies have shown an association between the observed increased signal intensity in the brain after repeated GBCA administrations and the occurrence of any adverse health effects [85], including in patients with normal renal function [70, 71].
Based on the recommendations of the European Medicines Agency, the European Commission has decided to restrict or suspend the marketing authorizations for the intravenous use of all multipurpose linear GBCAs (gadobenate dimeglumine, gadodiamide, gadopentetate dimeglumine and gadoversetamide) [86]. Thus, marketing authorizations for gadodiamide, gadopentetate dimeglumine and gadoversetamide were suspended, with use of gadobenate dimeglumine restricted to liver MRI, and gadopentetate dimeglumine restricted to intra-articular use for joint scans. This European Commission decision included updates to the prescribing information of all GBCAs remaining on the market, including macrocyclic GBCAs (gadobutrol, gadoteric acid and gadoteridol) and speciality linear GBCAs [gadoxetic acid (liver imaging), gadobenate dimeglumine (liver imaging) and gadopentetate dimeglumine (2 mmol/L intra-articular imaging)] [86]. The US FDA required a new class warning be added to the label of all GBCAs concerning retention of gadolinium in patients’ bodies for months to years after GBCA administration and also required other safety measures [87].
Gadobutrol has a very good overall safety profile in the clinical trial and real-world settings, with most adverse events of mild to moderate intensity and transient (Sect. 5). The safety profile of gadobutrol is similar in a diverse spectrum of patients and across all age groups, and similar to that of other GBCAs (Sect. 5). In keeping with its unique physicochemical profile (Sect. 2) and low propensity to be associated with NSF, after > 50 million applications of gadobutrol, only three patients met the criteria for being diagnostic of or consistent with NSF, and a possible association with gadobutrol could not be excluded (based on conservative worst-case scenario criteria) (Sect. 5.1).
In conclusion, its unique physicochemical profile means that gadobutrol is formulated at twice the Gd3+ ion concentration of other GBCAs, resulting in a narrower bolus and consequently, improved dynamic image enhancement. Based on > 20 years of experience in the clinical trial and real-world settings and its low risk for developing NSF, gadobutrol represents an effective and safe diagnostic GBCA for use in CE MRI and MRA to visualize pathological lesions and vascular perfusion and flow-related abnormalities in all body regions in a broad spectrum of patients, including term neonates and other paediatric patients, young and elderly adult patients, and those with moderate or severe renal or hepatic impairment or CV disease.
Data Selection Gadobutrol: 268 records identified
Duplicates removed | 65 |
Excluded during initial screening (e.g. press releases; news reports; not relevant drug/indication; preclinical study; reviews; case reports; not randomized trial) | 60 |
Excluded during writing (e.g. reviews; duplicate data; small patient number; nonrandomized/phase I/II trials) | 56 |
Cited efficacy/tolerability articles | 47 |
Cited articles not efficacy/tolerability | 40 |
Search Strategy: EMBASE, MEDLINE and PubMed from 2013 to present. Previous Adis Drug Evaluation published in 2013 was hand-searched for relevant data. Clinical trial registries/databases and websites were also searched for relevant data. Key words were Gadobutrol, Gadavist, Gadovist, MRI, imaging. Records were limited to those in English language. Searches last updated 1 July 2018 | |
Change history
10 September 2018
The article Gadobutrol: A Review in Contrast-Enhanced MRI and M
23 August 2018
The cell entry in the “Gadobenate dimeglumine” column, which previously read.
References
American College of Rheumatology. ACR manual on contrast media: version 10.3. 2017. https://www.acr.org. Accessed 29 Mar 2018.
Grobner T. Gadolinium: a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transpl. 2006;21(4):1104–8.
European Medicines Agency. Gadolinium-containing contrast agents and nephrogenic systemic fibrosis: long-term consequences of retention in human skin and bone. 2010. http://www.ema.europa.eu/. Accessed 29 Mar 2018.
US FDA. New warnings for using gadolinium-based contrast agents in patients with kidney dysfunction. 2015. http://www.fda.gov. Accessed 29 Mar 2018.
Scott LJ. Gadobutrol: a review of its use for contrast-enhanced magnetic resonance imaging in adults and children. Clin Drug Investig. 2013;33(4):303–14.
Endrikat J, Anzalone N. Gadobutrol in India: a comprehensive review of safety and efficacy. Magn Reson Insights. 2017;10:1178623x17730048.
Rohrer M, Bauer H, Mintorovitch J, et al. Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Invest Radiol. 2005;40(11):715–24.
Gutierrez JE, Koenig S, Breuer J. Overview on the efficacy and safety of gadbutrol: an MRI contrast agent for the CNS, body and vessels. Imaging Med. 2012;4(1):25–40.
Huppertz A, Rohrer M. Gadobutrol, a highly concentrated MR-imaging contrast agent: its physicochemical characteristics and the basis for its use in contrast-enhanced MR angiography and perfusion imaging. Eur Radiol. 2004;14(Suppl 5):M12–8.
Kramer JH, Arnoldi E, Francois CJ, et al. Dynamic and static magnetic resonance angiography of the supra-aortic vessels at 3.0 T. Invest Radiol. 2013;48(3):121–8.
Bayer HealthCare Pharmaceuticals Inc. Gadavist (gadobutrol) injection, for intravenous use: US prescribing information. 2016. http://labeling.bayerhealthcare.com. Accessed 1 Mar 2018.
Bayer plc. Summary of product characteristics, labelling and package leaflet: Gadovist 1.0 mmol/mL solution for injection. 2017. https://www.medicines.org.uk/emc/product/2876. Accessed 26 Jun 2018.
Kunze C, Mentzel HJ, Krishnamurthy R, et al. Pharmacokinetics and safety of macrocyclic gadobutrol in children aged younger than 2 years including term newborns in comparison to older populations. Invest Radiol. 2016;51(1):50–7.
Hahn G, Sorge I, Gruhn B, et al. Pharmacokinetics and safety of gadobutrol-enhanced magnetic resonance imaging in pediatric patients. Invest Radiol. 2009;44(12):776–83.
Tombach B, Bremer C, Reimer P, et al. Pharmacokinetics of 1 M gadobutrol in patients with chronic renal failure. Invest Radiol. 2000;35(1):35–40.
Tombach B, Bremer C, Reimer P, et al. Using highly concentrated gadobutrol as an MR contrast agent in patients also requiring hemodialysis: safety and dialysability. Am J Roentgen. 2002;178(1):105–9.
Glutig K, Bhargava R, Hahn G, et al. Safety of gadobutrol in more than 1000 pediatric patients: subanalysis of the GARDIAN study, a global multicenter prospective non-interventional study. Pediatr Radiol. 2016;46(9):1317–23.
Prince MR, Lee HG, Lee CH, et al. Safety of gadobutrol in over 23,000 patients: the GARDIAN study, a global multicentre, prospective, non-interventional study. Eur Radiol. 2017;27(1):286–95.
Bhargava R, Noga M. Safety and efficacy of gadobutrol-enhanced MRI in patients aged under 2 years: a single-center, observational study. Magn Reson Insights. 2013;6:1–12.
Gutierrez JE, Rosenberg M, Seemann J, et al. Safety and efficacy of gadobutrol for contrast-enhanced magnetic resonance imaging of the central nervous system: results from a multicenter, double-blind, randomized, comparator study. Magn Reson Insights. 2015;8:1–10.
Gutierrez JE, Rosenberg M, Duhaney M, et al. Phase 3 efficacy and safety trial of gadobutrol, a 1.0 molar macrocyclic MR imaging contrast agent, in patients referred for contrast-enhanced MR imaging of the central nervous system. J Magn Reson Imaging. 2015;41(3):788–96.
Anzalone N, Scarabino T, Venturi C, et al. Cerebral neoplastic enhancing lesions: multicenter, randomized, crossover intraindividual comparison between gadobutrol (1.0 M) and gadoterate meglumine (0.5 M) at 0.1 mmol Gd/kg body weight in a clinical setting. Eur J Radiol. 2013;82(1):139–45.
Tanaka A, Masumoto T, Yamada H, et al. A Japanese, multicenter, open-label, phase 3 study to investigate the safety and efficacy of gadobutrol for contrast-enhanced MR imaging of the central nervous system. Magn Reson Med Sci. 2016;15(2):227–36.
Katakami N, Inaba Y, Sugata S, et al. Magnetic resonance evaluation of brain metastases from systemic malignances with two doses of gadobutrol 1.0 M compared with gadoteridol: a multicenter, phase II/III study in patients with known or suspected brain metastases. Invest Radiol. 2011;46(7):411–8.
Maravilla KR, San-Juan D, Kim SJ, et al. Comparison of gadoterate meglumine and gadobutrol in the MRI diagnosis of primary brain tumors: a double-blind randomized controlled intraindividual crossover study (the REMIND Study). Am J Neuroradiol. 2017;38(9):1681–8.
Maravilla KR, Smith MP, Vymazal J, et al. Are there differences between macrocyclic gadolinium contrast agents for brain tumor imaging? Results of a multicenter intraindividual crossover comparison of gadobutrol with gadoteridol (the TRUTH study). Am J Neuroradiol. 2015;36(1):14–23.
Saake M, Langner S, Schwenke C, et al. MRI in multiple sclerosis: an intra-individual, randomized and multicentric comparison of gadobutrol with gadoterate meglumine at 3 T. Eur Radiol. 2016;26(3):820–8.
Hentsch A, Aschauer MA, Balzer JO, et al. Gadobutrol-enhanced moving-table magnetic resonance angiography in patients with peripheral vascular disease: a prospective, multi-centre blinded comparison with digital subtraction angiography. Eur Radiol. 2003;13(9):2103–14.
Schaefer FKW, Schaefer PJ, Altjohann C, et al. A multicenter, site-independent, blinded study to compare the diagnostic accuracy of contrast-enhanced magnetic resonance angiography using 1.0 M gadobutrol (Gadovist™) to intraarterial digital subtraction angiography in body arteries. Eur J Radiol. 2007;61(2):315–23.
Loewe C, Arnaiz J, Krause D, et al. MR angiography at 3 T of peripheral arterial disease: a randomized prospective comparison of gadoterate meglumine and gadobutrol. Am J Roentgenol. 2015;204(6):1311–21.
Hoelter P, Lang S, Weibart M, et al. Prospective intraindividual comparison of gadoterate and gadobutrol for cervical and intracranial contrast-enhanced magnetic resonance angiography. Neuroradiology. 2017;59(12):1233–9.
Hammerstingl R, Adam G, Ayuso J-R, et al. Comparison of 1.0 M gadobutrol and 0.5 M gadopentetate dimeglumine-enhanced magnetic resonance imaging in five hundred seventy-two patients with known or suspected liver lesions: results of a multicenter, double-blind, interindividual, randomized clinical phase-III trial. Invest Radiol. 2009;44(3):168–76.
Tombach B, Bohndorf K, Brodtrager W, et al. Comparison of 1.0 M gadobutrol and 0.5 M gadopentate dimeglumine-enhanced MRI in 471 patients with known or suspected renal lesions: results of a multicenter, single-blind, interindividual, randomized clinical phase III trial. Eur Radiol. 2008;18(11):2610–9.
Sardanelli F, Newstead GM, Putz B, et al. Gadobutrol-enhanced magnetic resonance imaging of the breast in the preoperative setting: results of 2 prospective international multicenter phase III studies. Invest Radiol. 2016;51(7):454–61.
Fallenberg EM, Renz DM, Karle B, et al. Intraindividual, randomized comparison of the macrocyclic contrast agents gadobutrol and gadoterate meglumine in breast magnetic resonance imaging. Eur Radiol. 2015;25(3):837–49.
Pediconi F, Kubik-Huch R, Chilla B, et al. Intra-individual randomised comparison of gadobutrol 1.0 M versus gadobenate dimeglumine 0.5 M in patients scheduled for preoperative breast MRI. Eur Radiol. 2013;23(1):84–92.
Escribano F, Sentis M, Oliva JC, et al. Dynamic magnetic resonance imaging of the breast: comparison of gadobutrol vs. Gd-DTPA. Radiologia. 2017;60(1):49–56.
Sardanelli F. Preoperative breast MRI: first results from the MIPA study [abstract]. Insights into Imaging. 2017;8(Suppl. 1):S491.
Trimboli RM, Di Leo G, Sacchetto D, et al. New insights into preoperative breast magnetic resonance imaging (MRI) from the multicentre individual patient analysis (MIPA) study [abstract]. Insights into Imaging. 2017;8(Suppl. 1):485.
Emaus MJ, Bakker MF, Peeters PHM, et al. MR imaging as an additional screening modality for the detection of breast cancer in women aged 50-75 years with extremely dense breasts: the DENSE trial study design. Radiology. 2015;277(2):527–37.
Kuwatsuru R, Takahashi S, Umeoka S, et al. A multicenter, randomized, controlled, single-blind comparison phase III study to determine the efficacy and safety of gadobutrol 1.0 M versus gadopentetate dimeglumine following single injection in patients referred for contrast-enhanced MRI of the body regions or extremities. J Magn Reson Imaging. 2015;41(2):404–13.
Rudolph A, Messroghli D, von Knobelsdorff-Brenkenhoff F, et al. Prospective, randomized comparison of gadopentetate and gadobutrol to assess chronic myocardial infarction applying cardiovascular magnetic resonance. BMC Med Imaging. 2015;15:55.
Doeblin P, Schilling R, Wagner M, et al. Intraindividual comparison of T1 relaxation times after gadobutrol and Gd-DTPA administration for cardiac late enhancement imaging. Eur J Radiol. 2014;83(4):660–4.
Wildgruber M, Stadlbauer T, Rasper M, et al. Single-dose gadobutrol in comparison with single-dose gadobenate dimeglumine for magnetic resonance imaging of chronic myocardial infarction at 3 T. Invest Radiol. 2014;49(11):728–34.
De Cobelli F, Esposito A, Perseghin G, et al. Intraindividual comparison of gadobutrol and gadopentetate dimeglumine for detection of myocardial late enhancement in cardiac MRI. AM J Radiol. 2012;198:809–16.
Liu D, Ma X, Liu J, et al. Quantitative analysis of late gadolinium enhancement in hypertrophic cardiomyopathy: comparison of diagnostic performance in myocardial fibrosis between gadobutrol and gadopentetate dimeglumine. Int J Cardiovasc Imaging. 2017;33(8):1191–200.
Kim CK, Park JJ, Park BK. Prostate diffusion-weighted imaging at 3T: effect of intravenous gadobutrol administration. Eur Radiol. 2016;26(5):1450–6.
Durmus T, Vollnberg B, Schwenke C, et al. Dynamic contrast enhanced MRI of the prostate: comparison of gadobutrol and Gd-DTPA. Rofo. 2013;185(9):862–8.
Choi TW, Lee JM, Kim JH, et al. Comparison of multidetector CT and gadobutrol-enhanced MR imaging for evaluation of small, solid pancreatic lesions. Korean J Radiol. 2016;17(4):509–21.
Data on file, Bayer AG., 2018.
Forsting M, Palkowitsch P. Prevalence of acute adverse reactions to gadobutrol–a highly concentrated macrocyclic gadolinium chelate: review of 14,299 patients from observational trials. Eur J Radiol. 2010;74(3):e186–92.
Endrikat J, Vogtlaender K, Dohanish S, et al. Safety of gadobutrol: results from 42 clinical phase II to IV studies and postmarketing surveillance after 29 million applications. Invest Radiol. 2016;51(9):537–43.
Voth M, Rosenberg M, Breuer J. Safety of gadobutrol, a new generation of contrast agents: experience from clinical trials and postmarketing surveillance. Invest Radiol. 2011;46(11):663–71.
Endrikat J, Schwenke C, Prince MR. Gadobutrol for contrast-enhanced magnetic resonance imaging in elderly patients: review of the safety profile from clinical trial, post-marketing surveillance, and pharmacovigilance data. Clin Radiol. 2015;70(7):743–51.
Palkowitsch P, Voth M. Summary of the safety data for gadobutrol and gadofosveset. Eur Radiol. 2009;18(Suppl. 5):E47–54.
Balzer JO, Loewe C, Davis K, et al. Safety of contrast-enhanced MR angiography employing gadobutrol 1.0 M as contrast material. Eur Radiol. 2003;13(9):2067–74.
Power S, Talbot N, Kucharczyk W, et al. Allergic-like reactions to the MR imaging contrast agent gadobutrol: a prospective study of 32 991 consecutive injections. Radiology. 2016;281(1):72–7.
Michaely HJ, Aschauer M, Deutschmann H, et al. Gadobutrol in renally impaired patients: results of the GRIP study. Invest Radiol. 2017;52(1):55–60.
Malik M, Hnatkova K, Schmidt A, et al. Correction for QT/RR hysteresis in the assessment of drug-induced QTc changes: cardiac safety of gadobutrol. Ann Noninvasive Electrocardiol. 2009;14:242–50.
Granata V, Cascella M, Fusco R, et al. Immediate adverse reactions to gadolinium-based MR contrast media: a retrospective analysis on 10,608 examinations. BioMed Res Internat. 2016;10:3918292.
Frenzel T, Lengsfeld P, Schirmer H, et al. Stability of gadolinium-based magnetic resonance imaging contrast agents in human serum at 37 °C. Invest Radiol. 2008;43(12):817–28.
Endrikat J, Dohanish S, Schleyer N, et al. 10 years of nephrogenic systemic fibrosis: a comprehensive analysis of nephrogenic systemic fibrosis received by a pharmaceutical company from 2001 to 2016. Invest Radiol. 2018. https://doi.org/10.1097/RLI.0000000000000462.
Girardi M, Kay J, Elston DM, et al. Nephrogenic systemic fibrosis: clinicopathological definition and workup recommendations. J Am Acad Dermatol. 2011;65:1095.e7–1106.e7.
Bayer Pharmaceutical Co., Ltd. Gadovist IV injection: Japanese prescribing information. 2017. http://www.pmda.go.jp. Accessed 12 Feb 2018.
George E, Guenette JP, Lee TC. Introduction to neuroimaging. Am J Med. 2018;131:346–56.
Fehlings MG, Martin AR, Tetreault LA, et al. A clinical practice guideline for the management of patients with acute spinal cord injury: recommendations on the role of baseline magnetic resonance imaging in clinical decision making and outcome prediction. Global Spine J. 2017;7(35):221S–30S.
Holloway BJ, Rosewarne D, Jones RG. Imaging of thoracic aortic disease. Br J Radiol. 2011;84(3):S338–54.
Sieber MA, Lengsfeld P, Frenzel T, et al. Preclinical investigation to compare different gadolinium-based contrast agents regarding their propensity to release gadolinium in vivo and to trigger nephrogenic systemic fibrosis-like lesions. Eur Radiol. 2008;18(10):2164–73.
European Society of Urogenital Radiology. ESUR guidelines on contrast media: version 8.1. 2017. http://www.esur.org/guidelines/. Accessed 23 Apr 2018.
Kanda T, Oba H, Toyoda K, et al. Brain gadolinium deposition after administration of gadolinium-based contrast agents. Jpn J Radiol. 2016;34(1):3–9.
Olchowy C, Cebulski K, Lasecki M, et al. The presence of the gadolinium-based contrast agent depositions in the brain and symptoms of gadolinium neurotoxicity: a systematic review. PLoS One. 2017;12(2):e0171704.
Cao Y, Huang DQ, Shih G, et al. Signal change in the dentate nucleus on T1-weighted MR images after multiple administrations of gadopentetate dimeglumine versus gadobutrol. Am J Roentgenol. 2016;206(2):414–9.
Radbruch A, Haase R, Kieslich PJ, et al. No signal intensity increase in the dentate nucleus on unenhanced T1-weighted MR images after more than 20 serial injections of macrocyclic gadolinium-based contrast agents. Radiology. 2017;282(3):699–707.
Radbruch A, Weberling LD, Kieslich PJ, et al. High-signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted images: evaluation of the macrocyclic gadolinium-based contrast agent gadobutrol. Invest Radiol. 2015;50(12):805–10.
Renz DM, Kumpel S, Bottcher J, et al. Comparison of unenhanced T1-weighted signal intensities within the dentate nucleus and the globus pallidus after serial applications of gadopentetate dimeglumine versus gadobutrol in a pediatric population. Invest Radiol. 2018;53(2):119–27.
Schlemm L, Chien C, Bellmann-Strobl J, et al. Gadopentetate but not gadobutrol accumulates in the dentate nucleus of multiple sclerosis patients. Mult Scler. 2017;23(7):963–72.
Yoo RE, Sohn CH, Kang KM, et al. Evaluation of gadolinium retention after serial administrations of a macrocyclic gadolinium-based contrast agent (gadobutrol): a single-institution experience with 189 patients. Invest Radiol. 2018;53(1):20–5.
Fingerhut S, Niehoff AC, Sperling M, et al. Spatially resolved quantification of gadolinium deposited in the brain of a patient treated with gadolinium-based contrast agents. J Trace Elem Med Biol. 2018;45:125–30.
Kanda T, Fukusato T, Matsuda M, et al. Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology. 2015;276(1):228–32.
McDonald JS, McDonald RJ, Jentoft ME, et al. Intracranial gadolinium deposition following gadodiamide-enhanced magnetic resonance imaging in pediatric patients: a case-control study. JAMA Pediatr. 2017;171(7):705–7.
McDonald RJ, McDonald JS, Kallmes DF, et al. Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology. 2015;275(3):772–82.
McDonald RJ, McDonald JS, Kallmes DF, et al. Gadolinium deposition in human brain tissues after contrast-enhanced MR imaging in adult patients without intracranial abnormalities. Radiology. 2017;285(2):546–54.
Murata N, Gonzalez-Cuyar LF, Murata K, et al. Macrocyclic and other non-group 1 gadolinium contrast agents deposit low levels of gadolinium in brain and bone tissue: preliminary results from 9 patients with normal renal function. Invest Radiol. 2016;51(7):447–53.
Roberts DR, Welsh CA, LeBel DP 2nd, et al. Distribution map of gadolinium deposition within the cerebellum following GBCA administration. Neurology. 2017;88(12):1206–8.
Tedeschi E, Caranci F, Giordano F, et al. Gadolinium retention in the body: what we know and what we can do. Radiol Med. 2017;122:589–600.
European Medicines Agency. EMA’s final opinion confirms restrictions on use of linear gadolinium agents in body scans. 2017. http://www.ema.europa.eu/. Accessed 23 May 2018.
US FDA. FDA drug safety communication: FDA warns that gadolinium-based contrast agents (GBCAs) are retained in the body; requires new class warning. 2018. http://www.fda.gov. Accessed 23 May 2018.
Funding
The preparation of this review was not supported by any external funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
During the peer review process, the manufacturer of gadobutrol was also offered an opportunity to review this article. Changes resulting from comments received were made on the basis of scientific and editorial merit.
Conflict of interest
Lesley Scott is a salaried employee of Adis/Springer, is responsible for the article content and declares no relevant conflicts of interest.
Additional information
The manuscript was reviewed by: J. Kashima, Department of Pathology, Tokyo Metropolitan Cancer and Infectious Diseases Center, Komagome Hospital, Tokyo, Japan; F. Pasi, Fondazione IRCSS Policinico San Matteo, Radiotherapy Oncology, Pavia Italy.
The original version of this article was revised: In Table 1, Ligand structure row/gadobenate dimeglumine column: The cell entry "Macrocyclic" in the “Gadobenate dimeglumine” column has been changed to “Linear”.
The original version of this article was revised due to a retrospective Open Access request.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
About this article
Cite this article
Scott, L.J. Gadobutrol: A Review in Contrast-Enhanced MRI and MRA. Clin Drug Investig 38, 773–784 (2018). https://doi.org/10.1007/s40261-018-0674-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-018-0674-9