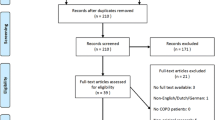
Abstract
Background and Objective
Chronic obstructive pulmonary disease (COPD), a progressive lung disorder associated with decline of respiratory function, affects 10.2% of Spanish adults (40–80 years of age). This study aimed to assess the cost-effectiveness of two fixed-dose combinations of long-acting muscarinic antagonist and long-acting β2-agonist therapies for COPD, with Spanish National Health System perspective.
Methods
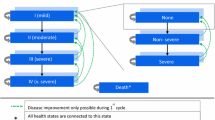
A Markov model with five health states based on severity levels defined by GOLD 2010 criteria was used to simulate in monthly cycles the evolution along a 5-year period of a cohort of moderate-to-severe COPD patients, treated with aclidinium–formoterol (ACL/FF) 400/12 µg or tiotropium–olodaterol (TIO/OLO) 5/5 µg fixed-dose combinations. Clinical data on lung-function improvement were obtained from a network meta-analysis and applied to mean baseline forced-expiratory-volume in 1 s (FEV1) for the first 24-weeks period. Natural history for lung-function decline (41 ml/year) was applied until the end of simulation. Risk of exacerbation and pneumonia occurrence were considered. Pharmaceutical costs were calculated with dosages according to indication and public ex-factory prices. The health state-specific disease management and event costs, and utilities were derived from the literature. Total costs (€ 2016) and benefits [life-year-gained (LYG) and quality-adjusted-life-year (QALY)] were discounted (3.0% yearly). Sensitivity analyses were performed.
Results
Both therapies provided the same outcomes (4.073 LYG and 2.928 QALY) at 5-year period. ACL/FF 400/12 µg provided marginally lower costs (€ − 332) compared to TIO/OLO 5/5 µg.
Conclusion
ACL/FF 400/12 µg was a cost-saving therapy in patients with moderate-to-severe COPD in Spain, and provided equivalent effects compared to TIO/OLO 5/5 µg.

Similar content being viewed by others
References
Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of COPD, global initiative for chronic obstructive lung disease (GOLD) 2014. http://www.goldcopd.org/. Accessed 28 Jul 2016.
Burge S, Wedzicha JA. COPD exacerbations: definitions and classifications. Eur Respir J. 2003;41(Suppl):S46–53.
Adeloye D, Chua S, Lee C, et al. Global and regional estimates of COPD prevalence: systematic review and meta-analysis. J Glob Health. 2015;5(2):020415.
Miravitlles M, Soriano JB, García-Río F, et al. Prevalence of COPD in Spain: impact of undiagnosed COPD on quality of life and daily life activities. Thorax. 2009;64(10):863–8.
Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for chronic obstructive pulmonary disease (GOLD). Workshop summary. Am J Respir Crit Care Med. 2013;187:347–65.
Ministerio de Sanidad y Política Social. Plan de Calidad para el Sistema Nacional de Salud. Estrategia en EPOC del Sistema Nacional de Salud. Sanidad 2009. Ministerio de Sanidad y Política Social. http://www.msc.es/organizacion/sns/planCalidadSNS/docs/EstrategiaEPOCSNS.pdf. Accessed 05 Apr 2017.
Sicras A, Huerta A, Navarro R, Ibañez J. Uso de recursos y costs asociados a las exacerbaciones de enfermedad pulmonar obstructive crónica: estudio retrospective de base poblacional. Semergen. 2014;40(4):189–97.
Atsou K, Chouaid C, Hejblum G. Variability of the chronic obstructive pulmonary disease key epidemiological data in Europe: systematic review. BMC Med. 2011;9:7.
Rycroft CE, Heyes A, Lanza L, Becker K. Epidemiology of chronic obstructive pulmonary disease: a literature review. Int J Chron Obstruct Pulmon Dis. 2012;7:457–94.
Miravitlles M, Soler-Cataluña JJ, Calle M, Guía Española de la EPOC (GesEPOC), et al. (GesEPOC). Tratamiento farmacológico de la EPOC estable. Arch Bronconeumol. 2012;48(7):247–57.
Tashkin DP, Ferguson GT. Combination bronchodilator therapy in the management of chronic obstructive pulmonary disease. Respir Res. 2013;14:49.
Banerji D, Mahler DA, Hanania NA. Efficacy and safety of LABA/LAMA fixed-dose combinations approved in the US for the management of COPD. Expert Rev Respir Med. 2016;10(7):767–80.
Calzetta L, Rogliani P, Matera MG, Cazzola M. A systematic review with meta-analysis of dual bronchodilation with LAMA/LABA for the treatment of stable COPD. Chest. 2016;149(5):1181–96.
Di Marco F, Santus P, Scichilone N, et al. Symptom variability and control in COPD: advantages of dual bronchodilation therapy. Respir Med. 2017;125:49–56.
Centro Vasco de Información de Medicamentos. Nuevos medicamentos inhalados en la EPOC: ¿qué nos aportan?. Boletín de información farmacoterapéutica de la comarca (INFAC). 2015;23(9). http://www.euskadi.eus/contenidos/informacion/cevime_infac_2015/es_def/adjuntos/INFAC_Vol_23_n_9_nuevos_medicamentos_inhalados.pdf. Accessed 06 Apr 2017.
Feldman GJ, Sousa AR, Lipson DA, et al. Comparative efficacy of once-daily umeclidinium/vilanterol and tiotropium/olodaterol therapy in sympomatic chronic obstructive pulmonary disease: a randomized study. Adv Ther. 2017;34(11):2518–33.
Feldman GJ, Edin A. The combination of umeclidinium bromide and vilanterol in the management of chronic obstructive pulmonary disease: current evidence and future prospects. Ther Adv Respir Dis. 2013;7(6):311–9.
Huisman EL, Cockle SM, Ismaila AS, Karabis A, Punekar YS. Comparative efficacy of combination bronchodilator therapies in COPD: a network meta-analysis. Int J Chron Obstruct Pulmon Dis. 2015;10:1863–81.
Schlueter M, Gonzalez-Rojas N, Baldwin M, Groenke L, Voss F, Reason T. Comparative efficacy of fixed-dose combinations of long-acting muscarinic antagonists and long-acting β2-agonists: a systematic review and network meta-analysis. Ther Adv Respir Dis. 2016;10(2):89–104.
Selya-Hammer C, Gonzalez-Rojas Guix N, Baldwin M, et al. Development of an enhanced health-economic model and cost-effectiveness analysis of tiotropium + olodaterol Respimat® fixed-dose combination for chronic obstructive pulmonary disease patients in Italy. Ther Adv Respir Dis. 2016;10(5):391–401.
Wilson MR, Patel JG, Coleman A, McDade CL, Stanford RH, Earnshaw SR. Cost-effectiveness analysis of umeclidinium/vilanterol for the management of patients with moderate to very severe COPD using an economic model. Int J Chron Obstruct Pulmon Dis. 2017;12:997–1008.
Miravitlles M, Gáldiz JB, Huerta A, Villacampa A, Carcedo D, Garcia-Rio F. Cost-effectiveness of combination therapy umeclidinium/vilanterol versus tiotropium in symptomatic COPD Spanish patients. Int J Chron Obstruct Pulmon Dis. 2016;11:123–32.
Caro JJ, Briggs AH, Siebert U, Kuntz KM. Modeling good research practices–overview: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-1. Value Health. 2012;15(6):796–803.
National Clinical Guideline Centre (2010). Chronic obstructive pulmonary disease: management of chronic obstructive pulmonary disease in adults in primary and secondary care. London: National Clinical Guideline Centre. http://guidance.nice.org.uk/CG101/Guidance/pdf/. Accessed 30 Aug 2016.
López-Bastida J, Oliva J, Antoñanzas F, García-Altés A, Gisbert R, Mar J, Puig-Junoy J. Spanish recommendations on economic evaluation of health technologies. Eur J Health Econ. 2010;11(5):513–20.
Rutten-van Mölken MP, Oostenbrink JB, Miravitlles M, Monz BU. Modelling the 5-year cost effectiveness of tiotropium, salmeterol and ipratropium for the treatment of chronic obstructive pulmonary disease in Spain. Eur J Health Econ. 2007;8(2):123–35.
Miravitlles M, Murio C, Guerrero T. Factors associated with relapse after ambulatory treatment of acute exacerbations of chronic bronchitis. DAFNE Study Group. Eur Respir J. 2001;17(5):928–33.
Bateman ED, Chapman KR, Singh D, et al. Aclidinium bromide and formoterol fumarate as a fixed-dose combination in COPD: pooled analysis of symptoms and exacerbations from two six-month, multicentre, randomised studies (ACLIFORM and AUGMENT). Respir Res. 2015;16:92.
D’Urzo AD, Rennard SI, Kerwin EM, Mergel V, Leselbaum AR, Caracta CF. Efficacy and safety of fixed-dose combinations of aclidinium bromide/formoterol fumarate: the 24-week, randomized, placebo-controlled AUGMENT COPD study. Respir Res. 2014;15:123.
Singh D, Jones PW, Bateman ED, et al. Efficacy and safety of aclidinium bromide/formoterol fumarate fixed-dose combinations compared with individual components and placebo in patients with COPD (ACLIFORM-COPD): a multicentre, randomised study. BMC Pulm Med. 2014;14:178.
Casanova C, Marin JM, Martinez-Gonzalez C, et al. COPD history assessment in SpaiN (CHAIN) Cohort. New GOLD classification: longitudinal data on group assignment. Respir Res. 2014;15:3.
Statistic National Institute. Instituto Nacional de Estadísitica. http://www.ine.es. Accessed 26 Aug 2016.
Tashkin DP, Celli B, Senn S, UPLIFT Study Investigators, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359(15):1543–54.
Ramos M, Haughney J, Henry N, Lindner L, Lamotte M. Cost versus utility of aclidinium bromide 400 µg plus formoterol fumarate dihydrate 12 µg compared to aclidinium bromide 400 µg alone in the management of moderate-to-severe COPD. Clin Outc Res. 2016;8:445–56.
Langhammer A, Johnsen R, Gulsvik A, Holmen TL, Bjermer L. Forced spirometry reference values for Norwegian adults: the Bronchial Obstruction in Nord–Trøndelag Study. Eur Respir J. 2001;18(5):770–9.
Miller DK, Homan SM. Determining transition probabilities: confusion and suggestions. Med Decis Mak. 1994;14(1):52–8.
Karabis A, Mocarski M, Eijgelshoven I, Bergman G. Economic evaluation of aclidinium bromide in the management of moderate to severe COPD: an analysis over 5 years. Clin Outc Res. 2014;6:175–85.
Oostenbrink JB, Rutten-van Mölken MP, Monz BU, FitzGerald JM. Probabilistic Markov model to assess the cost-effectiveness of bronchodilator therapy in COPD patients in different countries. Value Health. 2005;8(1):32–46.
Boutou AK, Shrikrishna D, Tanner RJ, et al. Lung function indices for predicting mortality in COPD. Eur Respir J. 2013;42(3):616–25.
Rutten-van Mölken MP, Oostenbrink JB, Tashkin DP, Burkhart D, Monz BU. Does quality of life of COPD patients as measured by the generic EuroQol five-dimension questionnaire differentiate between COPD severity stages? Chest. 2006;130(4):1117–28.
Decramer M, Celli B, Tashkin DP, et al. Clinical trial design considerations in assessing long-term functional impacts of tiotropium in COPD: the UPLIFT trial. COPD. 2004;1(2):303–12.
Decramer M, Celli B, Kesten S, Lystig T, Mehra S, Tashkin DP, UPLIFT Investigators. Effect of tiotropium on outcomes in patients with moderate chronic obstructive pulmonary disease (UPLIFT): a prespecified subgroup analysis of a randomised controlled trial. Lancet. 2009;374(9696):1171–8.
Spencer M, Briggs AH, Grossman RF, Rance L. Development of an economic model to assess the cost effectiveness of treatment interventions for chronic obstructive pulmonary disease. Pharmacoeconomics. 2005;23(6):619–37.
Paterson C, Langan CE, McKaig GA, et al. Assessing patient outcomes in acute exacerbations of chronic bronchitis: the measure your medical outcome profile (MYMOP), medical outcomes study 6-item general health survey (MOS-6A) and EuroQol (EQ-5D). Qual Life Res. 2000;9(5):521–7.
Consejo General de Colegios Oficiales de Farmacéuticos. Base de datos del Conocimiento Sanitario-Bot Plus 2.0 [Health Knowledge database-Bot Plus 2.0]. https://botplusweb.portalfarma.com/. Accessed 28 June 2016.
Ministerio de Sanidad, Servicios Sociales e Igualdad. Relación informativa de medicamentos afectados por las deducciones establecidas en el Real Decreto Ley 8/210 de 20 de Mayo por el que se adoptan medidas extraordinarias para la reducción del déficit público. [Internet]. Madrid: Ministerio de Sanidad, Servicios Sociales e Igualdad; 2010. https://www.msssi.gob.es/profesionales/farmacia/pdf/DeduccionesAgosto2016.pdf. Accessed 28 Aug 2016.
Agencia Española de Medicamentos y Productos Sanitarios. Ficha técnica de Spiolto® [Internet]. Madrid: Agencia Española de Medicamentos y Productos Sanitarios; 2013. https://www.aemps.gob.es/cima/pdfs/es/ft/79967/FichaTecnica_79967.html.pdf. Accessed 27 June 2016.
Agencia Europea de Medicamentos. Ficha técnica de Duaklir®. Londres: Agencia Europea de Medicamentos; 2011. http://www.ema.europa.eu/docs/es_ES/document_library/EPAR_-_Product_Information/human/003745/WC500178413.pdf. Accessed 27 June 2016.
Miravitlles M, Murio C, Guerrero T, Gisbert R. Decisiones sobre Antibioticoterapia y Farmacoeconomía en la EPOC. Pharmacoeconomic evaluation of acute exacerbations of chronic bronchitis and COPD. Chest. 2002;121(5):1449–55.
Miravitlles M, Murio C, Guerrero T, Gisbert R. Costs of chronic bronchitis and COPD: a 1-year follow-up study. Chest. 2003;123(3):784–91.
Oblikue Consulting. Base de datos de costes sanitarios eSalud [eSalud Health Cost database] [Internet]. http://www.oblikue.com/bbdcostes/. Accessed 29 June 2016.
Almagro P, Martinez-Camblor P, Soriano JB, et al. Finding the best thresholds of FEV1 and dyspnea to predict 5-year survival in COPD patients:the COCOMICS study. PLoS One. 2014;9(2):e89866.
Scanlon PD, Connett JE, Waller LA, et al. Lung Health Study Research Group. Smoking cessation and lung function in mild-to-moderate chronic obstructive pulmonary disease. The Lung Health Study. Am J Respir Crit Care Med. 2000;161(2 Pt 1):381–90.
Jansen JP, Fleurence R, Devine B, et al. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 1. Value Health. 2011;14(4):417–28.
Eklund O, Afzal F, Borgström F, Ojanguren ME, Crespo C, Baldwin M. Cost-effectiveness of tiotropium vs glycopyrronium in moderate to very severe Copd in Spain. Value Health. 2015;18(7):A501.
Tebboth A, Ternouth A, Gonzalez-Rojas N. UK-specific cost-effectiveness of tiotropium + olodaterol fixed-dose combination versus other LAMA + LABA combinations in patients with COPD. Clin Outc Res. 2016;8:667–74.
Laramée P, Millier A, Brodtkorb TH, et al. A comparison of Markov and discrete-time microsimulation approaches: simulating the avoidance of alcohol-attributable harmful events from reduction of alcohol consumption through treatment of alcohol dependence. Clin Drug Investig. 2016;36(11):945–95.
De Miguel Díez J. Farmacoeconomía en el asma y en la EPOC. Arch Bronconeumol. 2005;41(5):239–41.
Miravitlles M, Figueras M. El coste de la enfermedad pulmonar constructiva crónica en España. Opciones para una optimización de recursos. Arch Bronconeumol. 2001;37:388–93.
Bhardwaj SS, Camacho F, Derrow A, Fleischer AB Jr, Feldman SR. Statistical significance and clinical relevance: the importance of power in clinical trials in dermatology. Arch Dermatol. 2004;140(12):1520–3.
Van Dijk WD, van den Bemt L, van Weel C. Megatrials for bronchodilators in chronic obstructive pulmonary disease (COPD) treatment: time to reflect. J Am Board Fam Med. 2013;26(2):221–4.
Gillissen A, Buhl R, Kardos P, Puhan M, Rabe KF, Rothe T, Sauer R, Welte T, Worth H, Menz G. Trial end-point in chronic obstructive pulmonary disease(COPD): minimal clinically important difference. Pneumologie. 2008;62(3):149–55.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by AstraZeneca Spain.
Conflicts of interest
IO and MM are currently employed at PORIB, a consultant company specialized in economic evaluation of health interventions, which received financial support from AstraZeneca for the development of this study. CJA has received honoraria from Pharmacoeconomics and Outcomes Research Iberia for advocacy tasks related to this project. MC is an employee of AstraZeneca, Spain. LL is an employee of AstraZeneca, UK.
Rights and permissions
About this article
Cite this article
Capel, M., Mareque, M., Álvarez, C.J. et al. Cost-Effectiveness of Fixed-Dose Combinations Therapies for Chronic Obstructive Pulmonary Disease Treatment. Clin Drug Investig 38, 611–620 (2018). https://doi.org/10.1007/s40261-018-0646-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-018-0646-0