Abstract
Introduction
The management of advanced gastrointestinal stromal tumors (GISTs) has been modified considerably by the availability of costly tyrosine kinase inhibitors (TKIs); however, the best therapeutic sequence in terms of cost and effectiveness remains unknown.
Objective
The aim of this study was to compare four potential strategies (reflecting the potential daily practice), each including imatinib 400 mg/day, as first-line treatment: S1 (imatinib400/best supportive care [BSC]); S2 (imatinib400/imatinib800/BSC); S3 (imatinib400/sunitinib/BSC); and S4 (imatinib400/imatinib800/sunitinib/BSC).
Methods
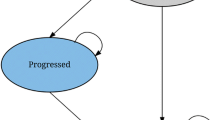
A Markov model was developed with a hypothetical cohort of patients and a lifetime horizon. Transition probabilities were estimated from the results of clinical trials. The analysis was performed from the French payer perspective, and only direct medical costs were included. Clinical and economic parameters were discounted, and the robustness of results was assessed.
Results
The least costly and effective strategy was S1, at a cost of €65,744 for 32.9 life months (reference). S3 was the most cost-effective strategy, with an incremental cost-effectiveness ratio (ICER) of €48,277/life-year saved (LYS). S2 was dominated, and S4 yielded an ICER of €363,320/LYS compared with S3. Sensitivity analyses confirmed the robustness of these results; however, when taking into account a price reduction of 80 % for imatinib, S2 and S4 become the most cost-effective strategies.
Conclusion
Our approach is innovative to the extent that our analysis takes into account the sequential application of TKIs. The results suggest that the S1 strategy is the best cost-effective strategy, but a price reduction of imatinib impacts on the results. This approach must continue, including new drugs and their impact on the quality of life of patients with advanced GISTs.


Similar content being viewed by others
References
Monges G, Bisot-Locard S, Blay JY, Bouvier AM, Urbieta M, Coindre JM, et al. The estimated incidence of gastrointestinal stromal tumors in France. Results of PROGIST conducted among pathologists. Bull Cancer. 2010;97(3):E16–22.
Blesius A, Cassier PA, Bertucci F, Fayette J, Ray-Coquard I, Bui B, et al. Neoadjuvant imatinib in patients with locally advanced non metastatic GIST in the prospective BFR14 trial. BMC Cancer. 2011;15:11–72.
Le Cesne A, Blay JY. Medical therapy of GIST: from palliative to curative treatment. Bull Acad Natl Med. 2012;196(4–5):861–74.
Dematteo RP, Heinrich MC, El-Rifai WM, Demetri G. Clinical management of gastrointestinal stromal tumors: before and after STI-571. Hum Pathol. 2002;33(5):466–77.
Van Oosterom AT, Judson I, Verweij J, Stroobants S, Donato di Paola E, Dimitrijevic S, et al. Safety and efficacy of imatinib (STI571) in metastatic gastrointestinal stromal tumours: a phase I study. Lancet. 2001;358(9296):1903.
Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347(7):472–80.
Blay JY, Le Cesne A, Ray-Coquard I, Bui B, Duffaud F, Delbaldo C, et al. Prospective multicentric randomized phase III study of imatinib in patients with advanced gastrointestinal stromal tumors comparing interruption versus continuation of treatment beyond 1 year: the French Sarcoma Group. J Clin Oncol. 2007;25(9):1107–13.
Blanke CD, Rankin C, Demetri GD, Ryan CW, von Mehren M, Benjamin RS, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26(4):626–32.
Verweij J, Casali PG, Zalcberg J, LeCesne A, Reichardt P, Blay JY, For the EORTC Soft Tissue and Bone Sarcoma Group, the Italian Sarcoma Group and the Australasian Gastrointestinal Trials Group, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomised trial. Lancet. 2004;364(9440):1127–34.
Gastrointestinal Stromal Tumor Meta-Analysis Group (MetaGIST). Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: a meta-analysis of 1640 patients. J Clin Oncol. 2010;28(7):1247–53.
Zalcberg JR, Verweij J, Casali PG, Le Cesne A, Reichardt P, Blay JY, et al. Outcome of patients with advanced gastro-intestinal stromal tumours crossing over to a daily imatinib dose of 800 mg after progression on 400 mg. Eur J Cancer. 2005;41(12):1751–7.
ESMO/European Sarcoma Network Working Group. Gastrointestinal stromal tumors: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii21–6.
National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology. Soft tissue sarcoma. 2015. Available at: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed 2 Sep 2016.
Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006;368(9544):1329–33.
Reichardt P, Kang Y, Ruka W. Detailed analysis of survival and safety with sunitinib (SU) in a worldwide treatment-use trial of patients with advanced GIST. J Clin Oncol. 2008;(Suppl 26):10548.
Demetri GD, Garrett CR, Schöffski P, Shah MH, Verweij J, Leyvraz S, et al. Complete longitudinal analyses of the randomized, placebo-controlled, phase III trial of sunitinib in patients with gastrointestinal stromal tumor following imatinib failure. Clin Cancer Res. 2012;18(11):3170–9.
Comptes nationaux de la santé 2013. Available at: http://www.drees.sante.gouv.fr/comptes-nationaux-de-la-sante-2013,11347.html. Accessed 2 Sep 2016.
Situation de la chimiothérapie des cancers–Rapport 2013. Collection état des lieux et des connaissances, ouvrage collectif édité par l’INCa, Boulogne-Billancourt, June 2014. Available at: http://www.e-cancer.fr/publications/71-soins/780-situation-de-la-chimiotherapie-des-cancers-rapport-2013 Accessed 2 Sep 2016.
Miller DK, Homan SM. Determining transition probabilities: confusion and suggestions. Med Decis Making. 1994;14(1):52–8.
Beck JR, Kassirer JP, Pauker SG. A convenient approximation of life expectancy (the “DEALE”): I. Validation of the method. Am J Med. 1982;73(6):883–88.
Beck JR, Pauker SG, Gottlieb JE, Klein K, Kassirer JP. A convenient approximation of life expectancy (the “DEALE”): II. Use in medical decision-making. Am J Med. 1982;73(6):889–97.
Haute Autorité de Santé, Department of Economics and Public Health Assessment. Choices in Methods for Economic Evaluation: a methodological guide. 2012. Available at: http://www.has-sante.fr. Accessed 2 Sep 2016.
Simoens S, Verbeken G, Huys I. Biosimilars and market access: a question of comparability and costs? Target Oncol. 2012;7:227–31.
Patrikidou A, Chabaud S, Ray-Coquard I, Bui BN, Adenis A, Rios M, et al. Influence of imatinib interruption and rechallenge on the residual disease in patients with advanced GIST: results of the BFR14 prospective French Sarcoma Group randomised, phase III trial. Ann Oncol. 2013;24(4):1087–93.
Debiec-Rychter M, Sciot R, Le Cesne A, Schlemmer M, Hohenberger P, van Oosterom AT, et al. KIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur J Cancer. 2006;43:1093–103.
Reichardt P, Kang YK, Rutkowski P, Schuette J, Rosen LS, Seddon B, et al. Clinical outcomes of patients with advanced gastrointestinal stromal tumors: safety and efficacy in a worldwide treatment-use trial of sunitinib. Cancer. 2015;121(9):1405–13.
Blanke CD, Huse DM. Cost effectiveness of tyrosine kinase inhibitor therapy in metastatic gastrointestinal stromal tumors. J Med Econ. 2010;13(4):681–90.
Huse DM, von Mehren M, Lenhart G, Joensuu H, Blanke C, Feng W, et al. Cost effectiveness of imatinib mesylate in the treatment of advanced gastrointestinal stromal tumours. Clin Drug Investig. 2007;27(2):85–93.
Mabasa VH, Taylor SC, Chu CC, Moravan V, Johnston K, Peacock S, et al. Verification of imatinib cost-effectiveness in advanced gastrointestinal stromal tumor in British Columbia (VINCE-BC study). J Oncol Pharm Pract. 2008;14(3):105–12.
Chabot I, LeLorier J, Blackstein ME. The challenge of conducting pharmacoeconomic evaluations in oncology using crossover trials: the example of sunitinib for gastrointestinal stromal tumour. Eur J Cancer. 2008;44(7):972–97.
Kang YK, Ryu MH, Yoo C, Ryoo BY, Kim HJ, Lee JJ, et al. Resumption of imatinib to control metastatic or unresectable gastrointestinal stromal tumours after failure of imatinib and sunitinib (RIGHT): a randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2013;14(12):1175–82.
Rochau U, Sroczynski G, Wolf D, Schmidt S, Jahn B, Kluibenschaedl M, et al. Cost-effectiveness of the sequential application of tyrosine kinase inhibitors for the treatment of chronic myeloid leukemia. Leuk Lymphoma. 2015;14:1–11.
Sullivan SD, Mauskopf JA, Augustovski F, Jaime Caro J, Lee KM, Minchin M, et al. Budget impact analysis-principles of good practice: report of the ISPOR 2012 Budget Impact Analysis Good Practice II Task Force. Value Health. 2014;17(1):5–14.
Appleby J, Devlin N, Parkin D. NICE’s cost effectiveness threshold. BMJ. 2007;335(7616):358–9.
Wilson J, Connock M, Song F, Yao G, Fry-Smith A, Raftery J, et al. Imatinib for the treatment of patients with unresectable and/or metastatic gastrointestinal stromal tumours: systematic review and economic evaluation. Health Technol Assess. 2005;9(25):1–142.
Paz-Ares L, García del Muro X, Grande E, González P, Brosa M, Díaz S. Cost-effectiveness analysis of sunitinib in patients with metastatic and/or unresectable gastrointestinal stroma tumours (GIST) after progression or intolerance with imatinib. Clin Transl Oncol. 2008;10(12):831–9.
Contreras-Hernández I, Mould-Quevedo JF, Silva A, Salinas-Escudero G, Villasís-Keever MA, Granados-García V, et al. A pharmaco-economic analysis of second-line treatment with imatinib or sunitinib in patients with advanced gastrointestinal stromal tumours. Br J Cancer. 2008;98(11):1762–8.
Poole CD, Connolly MP, Chang J, Currie CJ. Health utility of patients with advanced gastrointestinal stromal tumors (GIST) after failure of imatinib and sunitinib: findings from GRID, a randomized, double-blind, placebo-controlled phase III study of regorafenib versus placebo. Gastric Cancer. 2015;18(3):627–34.
George S, Von Mehren M, Heinrich MC, Corless CL, Zhu M, Butrynski JE, et al. A multicenter phase II study of regorafenib in patients (pts) with advanced gastrointestinal stromal tumor (GIST), after therapy with imatinib (IM) and sunitinib (SU). J Clin Oncol. 2012;30(19):2401–7.
Demetri GD, Reichardt P, Kang YK, Blay JY, Rutkowski P, Gelderblom H, et al. GRID study investigators. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):295–302.
Sawaki A, Nishida T, Doi T, Yamada Y, Komatsu Y, Kanda T, et al. Phase 2 study of nilotinib as third-line therapy for patients with gastrointestinal stromal tumor. Cancer. 2011;117(20):4633–41.
Reichardt P, Blay JY, Gelderblom H, Schlemmer M, Demetri GD, Bui-Nguyen B, et al. Phase III study of nilotinib versus best supportive care with or without a TKI in patients with gastrointestinal stromal tumors resistant to or intolerant of imatinib and sunitinib. Ann Oncol. 2012;23(7):1680–7.
Kindler HL, Campbell NP, Wroblewski K. Sorafenib (SOR) in patients (pts) with imatinib (IM) and sunitinib (SU)-resistant (RES) gastrointestinal stromal tumors (GIST): final results of a University of Chicago Phase II Consortium trial. J Clin Oncol. 2011;29(Suppl):10009.
Demetri GD, Heinrich MC, Chmielowski B. An open-label phase II study of the Hsp90 inhibitor ganetespib (STA-9090) in patients with metastatic and/or unresectable GIST. J Clin Oncol. 2011;29(suppl; abstr 10011).
Trent C, Wathen K, Von Mehren M. A phase II study of dasatinib for patients with imatinib-resistant gastrointestinal stromal tumor (GIST). J Clin Oncol. 2011;29(Suppl):10011.
Acknowledgments
The authors would like to thank Ms. Pamela Albert for her assistance in correcting the English language of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used in this study.
Conflict of interest
Virginie Nerich, Camille Fleck, Loïc Chaigneau, Nicolas Isambert, Christophe Borg, Elsa Kalbacher, Marine Jary, Pauline Simon, Xavier Pivot, Jean-Yves Blay, and Samuel Limat declare that they have no conflicts of interest and financial disclosures that are directly relevant to the content of this study.
Rights and permissions
About this article
Cite this article
Nerich, V., Fleck, C., Chaigneau, L. et al. Cost-Effectiveness Analysis of Tyrosine Kinase Inhibitors for Patients with Advanced Gastrointestinal Stromal Tumors. Clin Drug Investig 37, 85–94 (2017). https://doi.org/10.1007/s40261-016-0463-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-016-0463-2