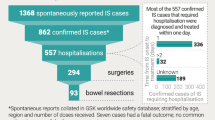
Abstract
Introduction
Two vaccines against rotavirus gastroenteritis (RVGE) in young children, Rotarix and RotaTeq, have been available in Europe since 2006. Vaccination against rotaviruses significantly reduces the burden of RVGE, but it is also associated with a very small increased risk of intussusception. In a benefit–risk analysis, the prevented RVGE burden is weighed against the possible excess of intussusception.
Purpose
The aim was to compare the estimated benefits and risks of Rotarix vaccination in France.
Methods
We estimated the benefits (vaccine-preventable RVGE hospitalizations and deaths) and risks (vaccine-caused intussusception hospitalizations and deaths) following two doses of Rotarix in a birth cohort of 791,183 followed for 3–5 years in France. We used data from peer-reviewed clinical and epidemiological studies or publications, and government statistics.
Results
Within the total number of French children below 5 years of age, we estimate vaccination could prevent a median 11,132 [95% credible interval (CI) 7842–14,408] RVGE hospitalizations and 7.43 (95% CI 3.27–14.68) RVGE deaths. At the same time, vaccination could cause an average of 6.86 (95% CI 2.25–38.37) intussusception hospitalizations and 0.0099 (95% CI 0.0024–0.060) intussusception deaths in the entire French birth cohort of infants below 1 year of age. Therefore, for every intussusception hospitalization and every intussusception death caused by vaccination, 1624 (95% CI 240–5243) RVGE hospitalizations and 743 (95% CI 93–3723) RVGE deaths are prevented, respectively, by vaccination.
Conclusions
The vaccine-prevented RVGE hospitalizations and deaths (benefit) greatly outweigh the excess potentially vaccination-related cases of intussusception (risk), indicating a favorable benefit–risk balance for Rotarix in France.
Similar content being viewed by others
Currently, rotavirus vaccination is not recommended in France due to safety concerns over intussusception caused by vaccination. |
We performed a benefit–risk analysis in France to compare estimated vaccine benefits (prevented gastroenteritis) with potential vaccine-related risks (excess intussusception). |
Our benefit–risk analysis predicts that the number of prevented rotavirus gastroenteritis hospitalizations and deaths would be 1624 [95% credible interval (CI) 240–5243] and 743 (95% CI 93–3723) times higher, respectively, than the excess intussusception hospitalizations or deaths potentially caused by vaccination. |
1 Introduction
Rotaviruses are the most common cause of severe diarrheal disease in young children throughout the world. Almost every child is infected at least once by the age of 3–5 years; globally, rotaviruses are the leading cause of severe dehydrating diarrhea in children under 5 years of age [1,2,3]. Prior to introduction of vaccination, rotavirus gastroenteritis (RVGE) accounted for approximately 2.4 million hospitalizations and 0.5 million deaths annually in this age category [2, 4,5,6]; in 2016, the World Health Organization (WHO) estimated that 215,000 child deaths occurred globally in 2013 due to rotavirus infection compared to 528,000 in 2000 [7]. Most of these deaths occur in developing countries, with about 90% of them in Africa and Asia [4, 7]. Although fatal outcomes are very rare in Europe, rotavirus disease imposes a considerable burden, especially on hospital services [8,9,10]. Furthermore, the highest rate of hospitalization due to RVGE occurs during the winter season (December to April). This peak in hospitalization rates has an important impact on healthcare services, because it coincides with the peak incidence of other diseases occurring in the winter season (e.g., respiratory tract infections caused by respiratory syncytial or influenza virus) [11]. Consequently, RVGE outbreaks contribute to the seasonal overcrowding of hospitals [10, 12,13,14,15].
Two live attenuated oral rotavirus vaccines (Rotarix, GSK, and RotaTeq, Merck & Co. Inc.) have been licensed and widely used in the past decade for the prevention of RVGE in young children. The vaccination course consists of two (Rotarix) or three (RotaTeq) oral doses. The first dose of both vaccines may be given at 6 weeks of age. The two doses of Rotarix should be separated by at least 4 weeks, and the vaccination course must be completed before the age of 24 weeks [16, 17]; all three doses of RotaTeq should be administered by 32 weeks of age. These vaccines have demonstrated high efficacy in randomized clinical trials within their development programs in the USA and European countries, including France [18,19,20,21]. Rotavirus vaccine effectiveness data have shown similar levels of protection against severe disease for Rotarix and RotaTeq [22]. Furthermore, a population-based prospective cohort study conducted in France using an active gastroenteritis surveillance system [23] found that vaccine introduction had a clinically relevant impact on hospitalization rates, in line with efficacy results from randomized trials. In 2013, WHO reissued recommendations to include Rotarix and RotaTeq in all national immunization programs [24].
Nevertheless, rotavirus vaccination may be associated with serious adverse events such as intussusception. Intussusception is observed when a segment of the intestine invaginates into an adjacent segment. Consequently, blood vessels are compressed, leading to pain, bowel edema, and eventually to intestinal ischemia, and even, with untreated cases where the arterial supply is compromised, to fatal outcomes [25]. Intussusception is a pediatric emergency. An average worldwide annual incidence of 74 cases per 100,000 infants (aged below 1 year) has been estimated [25]; in France, 25–50 cases of intussusception per 100,000 infants occur annually [26,27,28].
Data from epidemiological studies suggest that one to six excess cases of intussusception per 100,000 vaccinated infants might be attributable to rotavirus vaccination [29]. An increased risk of intussusception has been observed mostly within 7 days of vaccination following administration of the two first doses of Rotarix and RotaTeq [30,31,32,33,34,35,36]. However, it remains unclear whether rotavirus vaccines affect the overall incidence of intussusception based on longer periods of follow-up.
In Europe, Rotarix and RotaTeq received marketing authorization in 2006. Rotarix was introduced in France the same year, but the Haut Conseil de la Santé Publique (HCSP) decided to postpone the recommendation for universal rotavirus vaccination due to the absence of data for effectiveness and benefit–risk and cost–benefit ratios [37]. In 2010, the HCSP decided not to recommend vaccination because of safety and quality concerns (intussusceptions and contamination with porcine circovirus) and a lack of evidence of an effect on mortality [38]. In 2013, the HCSP reevaluated available data and recommended vaccination of all infants [26] on the basis of the positive effect of rotavirus vaccination programs in industrialized countries [15]. However, in 2014, the French Technical Committee for Pharmacovigilance reported two fatalities in French infants who had developed intussusception following vaccination between 2006 and 2012 [39]. In response to these deaths, in 2015, the Haute Autorité de Santé (HAS) decided to not reimburse rotavirus vaccination [40]. At the same time, the HCSP suspended the previous rotavirus vaccination recommendation [41].
Given the historical context in France, it is important to weigh the increased risk of intussusception against the benefits of reducing hospitalizations and deaths from rotavirus disease [42]. Benefit–risk analyses offer good insights into the value of vaccination and can be an important source of information for a variety of stakeholders, including regulatory authorities, clinicians and parents. A summary contextualizing the results and potential clinical research relevance and impact is displayed in the “Focus on the Patient” section (Fig. 1), for the benefit of health care professionals. Previous benefit–risk assessments performed in other regions of the world have shown that the benefits greatly outweigh the risks of rotavirus vaccination [32, 34, 43,44,45,46,47]. This benefit–risk analysis of rotavirus vaccination versus no-vaccination was performed over periods of 3 and 5 years in France, and compared vaccine-preventable hospitalizations and deaths due to RVGE (benefit) with vaccine-associated hospitalizations and deaths due to intussusception (risk) following two doses of Rotarix.
2 Methods
Data from peer-reviewed clinical and epidemiological studies, publications and national French statistics were used to conduct the benefit–risk analysis. The source data were identified, reviewed and validated by several GSK experts in epidemiology and safety. All parameters included in the benefit–risk analysis and their random distributions are provided in Table 1.
Benefit–risk estimates were calculated for a large number (~ 106) of scenarios generated randomly by selecting different combinations of values drawn from the distribution of the input parameters. Credible intervals (CIs) around the benefit–risk ratios and differences were calculated based on probabilistic uncertainty analyses and Monte-Carlo simulations [43, 48].
2.1 Data Used to Support Benefit Estimate
The burden of RVGE is relatively high in France, with a yearly estimate of 14,000 hospitalizations of children under 3 years of age, and a number of deaths between five and 13 over the 2007–2010 period [26]. The baseline RVGE hospitalization rate for children younger than 5 years was estimated based on the number of acute gastroenteritis cases in France over 11 seasons (1999–2010) in children younger than 3 years [26], using an integration method of the age-specific RVGE rate curve provided by Fourquet et al. [12]. The calculation of the age-specific RVGE preventable fraction of the RVGE incidence is described in Supplementary Figure S1 [see the Electronic Supplementary Material (ESM)]. Case-fatality ratios (67.5 deaths per 105 hospitalizations) were derived as an empirical distribution from the RVGE mortality rate observed in France and the RVGE hospitalization rate [12, 26, 49, 50]. The RVGE mortality rate for children below 3 years of age was estimated based on the HCSP data, which reported a minimum of four and maximum of 18 deaths over the 2007–2010 period [26]. The estimated value was extrapolated to children below 5 years of age. Baseline rates were calculated by including all children, independent of their vaccination status. The calculation of RVGE mortality rate included, for exhaustiveness purposes, deaths that occurred outside hospital environments.
No data on vaccine effectiveness in France were available at the time this study was conducted. For this reason, we used vaccine effectiveness estimates against RVGE after two vaccine doses from a study conducted in a neighboring country, Belgium (i.e., the results from a prospective case–control study in 39 Belgian hospitals) [51]; vaccine effectiveness post dose 1 was extrapolated for France using a ratio of vaccine effectiveness against RVGE after a one-dose compared to a two-dose schedule based on data from the USA and Brazil [52, 53]. We assumed the ratio of vaccine effectiveness to be constant across countries. Additionally, we assumed that vaccine effectiveness would apply identically for children below 3 and 5 years of age.
Several model parameters were derived from French data: (1) French birth cohort data were used as the denominator for the calculation of hospitalization and death incidence rates [54]. (2) An average value from 2006 to 2014 of 791,183 live births per year was used to estimate future cohorts, since there was little variation in the cohort size over this period. (3) The age distribution of children at first dose (median 2.18 months; 95% CI 1.42–5.11) and the delay before the second dose (median 1.10 months; 95% CI 0.78–2.50) were based on unpublished data from the Vaccinoscopie® study (see the ESM, Supplementary Figures S2 and S3). Vaccinoscopie® is an internet survey using a self-administrated questionnaire provided to a representative sample of mothers, and has monitored vaccine behaviors and the attitude of French parents since 2008 [55, 56]. The delay between the first and second dose was considered to be independent of the age of the child at first dose.
The age at the first dose of vaccine and the delay prior to the second dose of vaccine define the windows where the one-dose and the two-dose vaccine effectiveness apply and were used to calculate the vaccine-preventable fraction of the RVGE baseline rates. The reduction in the number of RVGE hospitalizations was calculated for the French birth cohort over a period of 3 and 5 years, and was derived from the baseline hospitalization rate and the vaccine-preventable fraction. We assumed that the reduction of risk of RVGE after one or two vaccine doses applies 2 weeks after injection.
Compliance to the second vaccine dose was estimated at 0.92 (95% CI 0.72–0.99) using the observed vaccine coverage in France for mandatory vaccination (diphtheria, tetanus and poliomyelitis) [57]. We assumed that compliance to the second vaccine dose was independent of any prognostic factor.
2.2 Data Used to Support Risk Estimate
Baseline intussusception hospitalization rates for infants < 1 year were estimated using 80 intussusception cases reported for eastern France in 2008 and 2009 by Fotso Kamdem et al. [27], together with the birth cohort for that region. We extrapolated this value to the birth cohort of metropolitan France. The age-specific baseline intussusception hospitalization rates were determined by combining data reported in two studies (Fotso Kamdem et al. and Serayssol et al.) [27, 28] and are presented in Supplementary Figure S4 (see the ESM). The intussusception mortality rate in France was calculated by estimating the number of intussusception deaths, identified in the Centre d’épidémiologie sur les causes médicales de décès (CEPI-DC) database [58] with the code K56.1 between 2000 and 2014 for infants < 1 year, and dividing by the sum of birth cohorts over the same period. The proportion of hospitalized infants with a fatal outcome was calculated by dividing the estimated overall intussusception mortality rate in France with the intussusception hospitalization rate. We assumed that the probability of a fatal outcome following intussusception hospitalization applies irrespective of the type and timing of hospitalization or the age of the child.
The intussusception hospitalization rates due to vaccination (i.e., the attributable risk) were calculated for each of the two vaccine doses as the average intussusception baseline hospitalization rate (calculated over the risk period following vaccination) multiplied by the relative risk of intussusception for each dose. The relative risk of intussusception following each dose was sourced from a meta-analysis across several countries (USA, Australia, Mexico, and Brazil) [30]; comparable relative risks were also described in another meta-analysis [31]. The increase in the number of intussusception hospitalizations after vaccination was calculated for a population of 105 vaccinees over a risk window of 7 days post doses 1 and 2. Additionally, we performed a sensitivity analysis on the risk window by extending the risk period to 21 and 30 days post vaccination, and considering the same risk as for the 7-day window.
2.3 Benefit Versus Risk Analysis
The hospitalization benefit–risk analysis was expressed as the ratio of the reduction in the number of severe RVGE hospitalizations attributable to vaccination over 3 or 5 years and the increase in the number of intussusception hospitalizations (deaths) attributable to vaccination for a risk window of 7 days following vaccination, relative to the same cohort of 105 (106) vaccinees. Similarly, the death benefit–risk comparison was expressed as a ratio of the reduction in the number of RVGE deaths over 3 or 5 years and the additional number of intussusception deaths observed over a risk window of 7 days post vaccination. The differences between benefits and risks were also estimated and expressed as the difference between the reduction in number of RVGE hospitalizations (or deaths) and the increase in number of intussusception hospitalizations (or deaths).
2.4 Statistical Methods
Random distributions were assigned to the key model parameters (Table 1) to implement up to 106 Monte-Carlo simulations and generate an empirical distribution for the ratio and difference of the benefit and risk estimates. Parameters of random distributions were estimated to match the 95% CI limits reported in publications, using conjugate Bayesian distributions and least-squares methods. In the absence of CI limits, the maximum and minimum values mentioned were used as 95% CI limits. Non-linear regression techniques were applied to calculate the RVGE and intussusception rates as a function of the age of children. Functional forms of the curves are derived from modifications of double-exponential models that appear to fit well to similar data across various countries. The sensitivity of the model for each input parameter was assessed comparing the benefit–risk estimate derived by using mean input values with the estimates derived by using the whole range of plausible input values. The variations around the mean benefit–risk estimates through simulations were correlated to the percentiles of the distribution of the key input parameters.
The CIs for the benefit–risk ratios were determined using the 2.5 and 97.5% percentiles of the empirical distributions. The medians are presented in the main body of this paper, as they are robust centrality estimates, less sensitive to extreme values or skewness of the distribution; at most, half of the simulations provide results less than the median value.
An outline of the benefit–risk model was explored in Excel, and specifications were written prior to implementation of the simulations in SAS software (version 9.2).
3 Results
In the absence of a rotavirus vaccine program in France, we estimated a median incidence of 15,059 (95% CI 12,100–18,476) RVGE hospitalizations and 10.13 (95% CI 4.64–19.46) RVGE deaths for an average French birth cohort of 791,183 children followed from birth to 5 years of age. Within the same cohort, we estimated an annual number of 323 (95% CI 257–400) intussusception hospitalizations and 0.45 (95% CI 0.19–0.88) intussusception deaths in infants below 1 year of age. All input parameters for these estimates are reported in Table 1.
The results following vaccination with two doses of Rotarix are given in Table 2. We estimate that vaccination would prevent approximately 75% of all RVGE hospitalizations and deaths in the total number of French children below 5 years of age, leading to a reduction of 11,132 (95% CI 7842–14,408) hospitalizations and 7.43 (95% CI 3.27–14.68) deaths. We also estimated that vaccination would cause 6.86 (95% CI 2.25–38.37) intussusception hospitalizations and 0.0099 (95% CI 0.0024–0.060) intussusception deaths in one French birth cohort of infants below 1 year of age. On average, one additional intussusception death caused by vaccination would be observed every 100 years if the birth cohort remains equal to 791,183 children.
The results of the probabilistic sensitivity analyses on the reduction in number of RVGE hospitalizations and deaths (benefit), the increase in number of intussusception hospitalizations and deaths (risk) and a ratio between benefit and risk, calculated for each simulation, are presented in Fig. 2. The Rotarix benefit–risk ratio for hospitalization is 1624 (95% CI 240–5243) for children below 5 years of age (Table 2), with 100% of the plausible values for the ratios, across all scenarios considered, being greater than ~ 48. This means that for each intussusception hospitalization caused, 1624 RVGE hospitalizations would be prevented, on average, by vaccination. Similarly, for each intussusception death caused, 743 (95% CI 93–3723) RVGE deaths would be prevented by vaccination, with 100% of the plausible values for the ratios, across all scenarios considered, being greater than ~ 8. Benefit–risk ratios in children below 3 years of age were similar (Table 2), with all plausible ratios, across all scenarios, being greater than ~ 48 for RVGE hospitalizations and greater than ~ 7 for RVGE deaths.
Two-dimensional plots of the overall reduction in the number of RVGE hospitalizations (a, b) and deaths (c, d) over 3 (a, c) and 5 years (b, d) risk windows (x axis) and the increase in numbers of IS hospitalizations (a, b) and deaths (c, d) post vaccination over two 7-day risk windows (y axis) for cohorts of 105 (hospitalization) and 106 (death) vaccinated French children. Each point represents the joint calculations of benefits and risks under a specific scenario selected at random from each of the random distributions of the input parameters. The results presenting the highest frequencies across the 106 simulations are colored in red. The plots illustrate the dominance of the hospitalization and death benefits in comparison to the hospitalization and death risks under all scenarios. D1 dose 1, D2 dose 2, hosp. hospitalization, IS intussusception, RVGE rotavirus gastroenteritis, sub. subjects
The sensitivity analyses summarized in tornado diagrams (Fig. 3) show the large influence of age at first dose on the benefit–risk ratio for hospitalization. The benefit–risk ratio deteriorates rapidly as the age at first dose gets closer to 6 months. On the other hand, the benefit–risk ratio increases greatly when the first dose is given very early (as soon as 1 month). The other impact parameters have much less influence on the benefit–risk ratio. The relative risk of intussusception following the second vaccine dose is the second most influential variable on the benefit–risk hospitalization ratio, but it has a much smaller influence than the age at first vaccination (Fig. 3a). In analyses on the benefit–risk death ratio, the fatality rates for intussusception and RVGE are the parameters with the greatest impact after age at first vaccination (Fig. 3b).
Sensitivity analysis assessing the impact of the variability of model parameters on the BR ratio for hospitalization (a) and death (b) in children 0–3 years of age. The tornado diagrams show on the x axis the variations of the BR ratio around its mean value because of variations of the main input parameters. The vertical line on the left part of the diagrams represents a BR ratio equal to 1. The left and right limits of each horizontal bar indicate the change in BR ratio calculated for the 1 and 99% percentile values of the input parameter mentioned. Other symbols indicate the BR variations expected for those percentiles of the input parameter. BR benefit–risk, D1 dose 1, D2 dose 2, IS intussusception, Max Maximum, Min Minimum, Q1 first quartile, Q3 third quartile, RR relative risk, RVGE rotavirus gastroenteritis, VE vaccine efficacy
4 Discussion
The present analysis was carried out to compare numerically the clinical benefits and risks associated with Rotarix vaccination in France. For a French birth cohort of 791,183 children vaccinated with two doses of Rotarix and followed for 5 years, an estimated 11,132 from the 15,059 hospitalizations observed in this population would be prevented (75% reduction), while causing a 6.9 excess of intussusception hospitalizations (one in every 114,942 vaccinated infants), which represents a 2% increase from the 323 annual baseline intussusception hospitalizations. Moreover, in the same birth cohort, we estimated that 7.43 RVGE deaths would be avoided in 5 years, while vaccination could possibly cause 0.01 deaths (which also represents a 2% increase over the baseline intussusception deaths). This analysis suggests that, on average, 1624 (95% CI 240–5243) RVGE-associated hospitalizations and 743 (95% CI 93–3723) RVGE-associated deaths would be prevented for each intussusception hospitalization and death caused, respectively.
Overall, our results are comparable to data from other benefit–risk studies (Table 3) [32, 34, 43,44,45, 47]. Geographical differences in the epidemiology of RVGE and intussusception and variations in the ranges of the modeling input values could explain the differences in the results between the various studies. However, all the studies published so far show that the benefits of vaccination would greatly outweigh the risks. The benefit–risk ratio for deaths in France is about twice that observed in Japan, but due to its variability (95% CI 93–3723), this difference is not significant. The benefit–risk ratio for hospitalizations is in the same range as the ratios from Brazil or the USA. Although the mean incidence of excess intussusception estimated in our study is low, the benefit–risk ratio remains favorable over the whole range of values (95% CI 0.0030–0.0754), which considers incidence of excess intussusception values up to six times higher than the median values.
RVGE can be a very serious condition, but in industrialized countries, improvements in hygiene and RVGE management practices (particularly oral rehydration therapy) have considerably reduced the burden of disease [59]. However, rotavirus transmission is difficult to control even with good primary hygienic measures [60]. In France, the probability of a fatal outcome following hospitalization for RVGE is relatively low (67.5/105 hospitalizations) and comparable with that of other European countries [8, 61]. Moreover, the RVGE probability of fatalities was shown to decrease in France between 2000 and 2010, while the number of hospitalizations was almost constant over the same period [62]. Even if the RVGE mortality rate in France decreased and reached a plateau in 2010, we think it can be further decreased with vaccination. This reduction would even be more visible for hospitalization related to RVGE. Our analysis predicts a ~ 75% decrease in the number of RVGE-related hospitalizations and deaths in children vaccinated with two doses of Rotarix.
Intussusception is the most frequent cause of acute intestinal obstruction among young children, regardless of exposure to rotavirus vaccines. Peak incidences occur in infants between 4 and 7 months of age [25, 26]. When treated early, intussusception has a good outcome and is rarely fatal. In France, both the yearly hospitalization rate (41/105 infants) and the case fatality ratio (139/105 hospitalizations) of intussusception are low. However, case fatality ratios for intussusception cases that are not hospitalized may be much higher. Moreover, a very recent study reported a trend towards decreasing intussusception incidence between 2009 and 2012 in France [26], but the authors could not identify a plausible explanation and suggested this might just be a natural variation in the rate of intussusception. Even though the French setting did provide two cases of deaths temporally related to intussusception since rotavirus vaccines were introduced, our analysis predicts a very low excess number of intussusception cases post vaccination: 6.9 hospitalizations and 0.01 deaths in the entire French birth cohort. We estimated the annual baseline incidence of intussusception death to be 0.45 in France between 2000 and 2014, and we did not observe an increase over this period in the CEPI-DC database [58]. Furthermore, the small risk of a fatal outcome following intussusception could be further decreased by appropriately informing the relevant health staff about the small increased risk of intussusception mostly during the 7 days after rotavirus vaccination, when rotavirus vaccines carry an increased risk of intussusception. There is limited evidence of a smaller increased risk following the second vaccine dose. Additionally, parents and caregivers should be adequately counseled to monitor the signs of intussusception and to seek immediate medical evaluation and treatment, which is key for intussusception management; not following these guidelines is probably one of the explanations for the two French death cases [39, 41].
The results of our sensitivity analyses regarding the influence of input parameters on the benefit–risk ratios are comparable to those previously described for Japan [47]. For the benefit–risk ratios, the most influential parameters are the ones that affect the risks (excess intussusception). Our results show that the benefit–risk hospitalization and deaths ratios would improve by 5000 and 3000 units, respectively, if the age at first vaccination decreased from its median value of 2.2 months. On the contrary, the benefit–risk ratio deteriorates rapidly as the age at first dose gets closer to 6 months.
We did not consider the risk of intussusception beyond the 7-day post-vaccination period since our analysis is based on relative risk estimates from a recent meta-analysis that only considered the risks over the 7-day period post vaccination [30], as was also the case in the other meta-analysis [31], mainly because of heterogeneity in the definition of other risk periods among various studies [30, 31]. Some studies have shown a small increased risk outside the 7-day post-vaccination window. In Mexico, an increased risk of intussusception by a factor of 2 was estimated during the 8- to 21-day period post dose 2 of Rotarix [32]; and in Australia, the relative incidence of intussusception was estimated to be three times higher during the 8- to 21-day period post dose 1 of Rotarix [34]. However, the benefit–risk ratios in France that resulted from the sensitivity analysis performed for 21 and 30 days post vaccination remained favorable, showing that an extended risk window does not alter the overall outcome of our assessment (see the ESM, Supplementary Table S1).
We only focused on the direct reduction of RVGE hospitalizations and deaths and did not take into consideration the induced herd protection and the possible impact of vaccination on nosocomial rotavirus infections, which could increase the benefit of vaccination. A recent study in Belgium (RotaBIS) [63] has shown that vaccination against rotavirus reduced the nosocomial infections by 85% between 2005 and 2012 and decreased the duration of hospital stays by 2 days, on average, per rotavirus event; the vaccine also induced herd protection across the different unvaccinated age-groups [10].
There are several limitations to this analysis: (1) there were no data in France to ascertain vaccine effectiveness and safety (these were nevertheless derived from valid sources and similar settings); (2) we did not attempt to compare the clinical severity or complications of RVGE and intussusception hospitalizations; (3) all RVGE or intussusception deaths are assumed to occur following hospitalization, and therefore, the reduction in RVGE hospitalizations through vaccination applies similarly to the reduction in RVGE deaths; (4) no uncertainty was propagated on the parameters of the curves used to described the age-specific RVGE or intussusception rates. The uncertainty was only propagated from the overall baseline rates. In contrast, some variability in the timing of the peak incidence rate was observed in the RVGE rate between Fourquet et al. and Huet et al. [12, 50], but was limited to a 1-month difference. Some of that variability may be due to the experimental conditions of those two studies.
5 Conclusion
This benefit–risk analysis is a tool that may help compare the benefits and risks of rotavirus vaccination in the French setting. Our modeling confirmed that the benefit–risk balance for Rotarix is favorable in France in the sense that the numbers of vaccine-prevented RVGE hospitalizations and deaths outweigh those potentially caused by intussusception, and therefore, vaccination should be considered a useful intervention for prevention.
References
Sanderson C, Clark A, Taylor D, Bolanos B. Global review of rotavirus morbidity and mortality data by age and region. In: Report to WHO/IVB. 2011. http://www.who.int/immunization/sage/meetings/2012/april/Sanderson_et_al_SAGE_April_rotavirus.pdf?ua=1. Accessed 10 Oct 2017.
Parashar UD, Hummelman EG, Bresee JS, Miller MA, Glass RI. Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis. 2003;9(5):565–72.
Lanata CF, Fischer-Walker CL, Olascoaga AC, Torres CX, Aryee MJ, Black RE. Global causes of diarrheal disease mortality in children < 5 years of age: a systematic review. PLoS ONE. 2013;8(9):e72788.
Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(2):136–41.
Parashar UD, Gibson CJ, Bresee JS, Glass RI. Rotavirus and severe childhood diarrhea. Emerg Infect Dis. 2006;12(2):304–6.
Parashar UD, Burton A, Lanata C, Boschi-Pinto C, Shibuya K, Steele D, et al. Global mortality associated with rotavirus disease among children in 2004. J Infect Dis. 2009;200(Suppl 1):S9–15.
van Zeijl JH, Mullaart RA, Galama JM. The pathogenesis of febrile seizures: is there a role for specific infections? Rev Med Virol. 2002;12(2):93–106.
Soriano-Gabarro M, Mrukowicz J, Vesikari T, Verstraeten T. Burden of rotavirus disease in European Union countries. Pediatr Infect Dis J. 2006;25(Suppl 1):S7–11.
Forster J, Guarino A, Parez N, Moraga F, Roman E, Mory O, et al. Hospital-based surveillance to estimate the burden of rotavirus gastroenteritis among European children younger than 5 years of age. Pediatrics. 2009;123(3):e393–400.
Standaert B, Strens D, Alwan A, Raes M. Medium- to long-term impact of rotavirus vaccination on hospital care in Belgium: a 7-year follow-up of the Rotavirus Belgium Impact Study (RotaBIS). Infect Dis Ther. 2016;5(1):31–44.
Le Roux P, Marshall B, Toutain F, Mary JF, Pinon G, Briquet E, et al. Nosocomial viral infections in a pediatric service: example of rotaviral gastroenteritis and respiratory syncytial viral bronchiolitis. Arch Pediatr. 2004;11(8):908–15.
Fourquet F, Desenclos JC, Maurage C, Baron S. Acute gastro-enteritis in children in France: estimates of disease burden through national hospital discharge data. Arch Pediatr. 2003;10(10):861–8.
Armengaud JB, El Hajje MJ, Moulin F, Marc E, Chalumeau M, Lebon P, et al. Simultaneous outbreaks of rotavirus and respiratory syncytial virus in Paris: a 12-year survey. Med Mal Infect. 2007;37(5):262–5.
Van Damme P, Giaquinto C, Huet F, Gothefors L, Maxwell M, Van der Wielen M. Multicenter prospective study of the burden of rotavirus acute gastroenteritis in Europe, 2004–2005: the REVEAL study. J Infect Dis. 2007;195(Suppl 1):S4–16.
Parez N, Giaquinto C, Du Roure C, Martinon-Torres F, Spoulou V, Van Damme P, et al. Rotavirus vaccination in Europe: drivers and barriers. Lancet Infect Dis. 2014;14(5):416–25.
MacDonald SE, Dover DC, Simmonds KA, Svenson LW. Risk of febrile seizures after first dose of measles–mumps–rubella–varicella vaccine: a population-based cohort study. CMAJ. 2014;186(11):824–9.
STIKO. Statement of the German Standing Committee on Vaccination at the RKI. Recommendations of the Standing Committee on Vaccination (STIKO) at the Robert Koch Institute–2017/2018. Epidemiol Bull. 2017;34:333–76.
Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354(1):23–33.
Vesikari T, Karvonen A, Prymula R, Schuster V, Tejedor JC, Cohen R, et al. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised, double-blind controlled study. Lancet. 2007;370(9601):1757–63.
Patel MM, Parashar UD, Santosham M, Richardson V. The rotavirus experience in Mexico: discovery to control. Clin Infect Dis. 2013;56(4):548–51.
Linhares AC, Justino MC. Rotavirus vaccination in Brazil: effectiveness and health impact seven years post-introduction. Expert Rev Vaccines. 2014;13(1):43–57.
Leshem E, Lopman B, Glass R, Gentsch J, Banyai K, Parashar U, et al. Distribution of rotavirus strains and strain-specific effectiveness of the rotavirus vaccine after its introduction: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14(9):847–56.
Gagneur A, Nowak E, Lemaitre T, Segura JF, Delaperriere N, Abalea L, et al. Impact of rotavirus vaccination on hospitalizations for rotavirus diarrhea: the IVANHOE study. Vaccine. 2011;29(21):3753–9.
Rotavirus vaccines. WHO position paper–—January 2013. Wkly Epidemiol Rec. 2013;88(5):49–64.
Jiang J, Jiang B, Parashar U, Nguyen T, Bines J, Patel MM. Childhood intussusception: a literature review. PLoS ONE. 2013;8(7):e68482.
Fotso Kamdem A, Vidal C, Pazart L, Leroux F, Pugin A, Savet C, et al. Incidence of acute intussusception among infants in eastern France: results of the EPIstudy trial. Eur J Pediatr. 2017;176(3):301–9.
Fotso Kamdem A, Vidal C, Pazart L, Leroux F, Savet C, Cornet C, et al. Épidémiologie de l’invagination intestinale aiguë chez l’enfant de moins de 1 an. Résultats préliminaires de l’étude Epistudy. Bull Epidémiol Hebd. 2012;10–11:138–43.
Serayssol C, Abbo O, Mouttalib S, Claudet I, Labarre D, Galinier P, et al. Seasonal pattern of intussusceptions in infants and children: is fall/winter predominance still worth consideration? A 10-year retrospective epidemiological study. Arch Pediatr. 2014;21(5):476–82.
Rha B, Tate JE, Weintraub E, Haber P, Yen C, Patel M, et al. Intussusception following rotavirus vaccination: an updated review of the available evidence. Expert Rev Vaccines. 2014;13(11):1339–48.
Rosillon D, Buyse H, Friedland LR, Ng SP, Velazquez FR, Breuer T. Risk of intussusception after rotavirus vaccination: meta-analysis of postlicensure studies. Pediatr Infect Dis J. 2015;34(7):763–8.
Dong R, Yang Y-F, Chen G, Shen Z, Zheng S. Risk of intussusception after rotavirus vaccination: a meta-analysis. Int J Clin Exp Med. 2016;9(2):1306–13.
Patel MM, Lopez-Collada VR, Bulhoes MM, De Oliveira LH, Bautista Marquez A, Flannery B, et al. Intussusception risk and health benefits of rotavirus vaccination in Mexico and Brazil. N Engl J Med. 2011;364(24):2283–92.
Velazquez FR, Colindres RE, Grajales C, Hernandez MT, Mercadillo MG, Torres FJ, et al. Postmarketing surveillance of intussusception following mass introduction of the attenuated human rotavirus vaccine in Mexico. Pediatr Infect Dis J. 2012;31(7):736–44.
Carlin JB, Macartney KK, Lee KJ, Quinn HE, Buttery J, Lopert R, et al. Intussusception risk and disease prevention associated with rotavirus vaccines in Australia’s national immunization program. Clin Infect Dis. 2013;57(10):1427–34.
Weintraub ES, Baggs J, Duffy J, Vellozzi C, Belongia EA, Irving S, et al. Risk of intussusception after monovalent rotavirus vaccination. N Engl J Med. 2014;370(6):513–9.
Yih WK, Lieu TA, Kulldorff M, Martin D, McMahill-Walraven CN, Platt R, et al. Intussusception risk after rotavirus vaccination in U.S. infants. N Engl J Med. 2014;370(6):503–12.
Avis du Conseil Supérieur d’Hygiène Publique de France; Section Maladies Transmissibles; Relatif à la vaccination anti-rotavirus chez les nourrissons de moins de six mois. 22 September and 05 December 2006 http://www.hcsp.fr/docspdf/cshpf/a_mt_220906_rotavirus.pdf. Accessed 12 Aug 2016.
Haut Conseil de la santé publique (HCSP). AVIS relatif à la vaccination contre le rotavirus des nourrissons de moins de 6 mois. 28 May 2010. http://www.hcsp.fr/Explore.cgi/avisrapportsdomaine?clefr=151. Accessed 12 Aug 2016.
Agence Nationale de Sécurité des Médicaments et des produits de santé (ANSM), Compte rendu de séance, Réunion du Comité technique de Pharmacovigilance – CT012015023. 10 février 2015. http://ansm.sante.fr/content/download/75433/958819/version/2/file/CR-CT-Pharmacovigilance-012015023+rotavirus.pdf. Accessed 12 Aug 2016.
Haute Autorité de Santé (HAS), Commission de la Transparence, Rotarix Avis CT13564. 1 April 2015. http://www.has-sante.fr/portail/upload/docs/evamed/CT-13564_ROTARIX_PIC_INS_Avis3_CT13564.pdf. Accessed 12 Aug 2016.
Haut Conseil de la santé publique (HCSP), Avis relatif aux infections à rotavirus : suspension des recommandations de vaccination des nourrissons. 21 April 2015. http://www.hcsp.fr/explore.cgi/avisrapportsdomaine?clefr=501 Accessed 12 Aug 2016.
Parashar UD, Cortese MM, Payne DC, Lopman B, Yen C, Tate JE. Value of post-licensure data on benefits and risks of vaccination to inform vaccine policy: the example of rotavirus vaccines. Am J Prev Med. 2015;49(6 Suppl 4):S377–82.
Desai R, Parashar UD, Lopman B, de Oliveira LH, Clark AD, Sanderson CF, et al. Potential intussusception risk versus health benefits from rotavirus vaccination in Latin America. Clin Infect Dis. 2012;54(10):1397–405.
Desai R, Cortese MM, Meltzer MI, Shankar M, Tate JE, Yen C, et al. Potential intussusception risk versus benefits of rotavirus vaccination in the United States. Pediatr Infect Dis J. 2013;32(1):1–7.
Clark A, Jit M, Andrews N, Atchison C, Edmunds WJ, Sanderson C. Evaluating the potential risks and benefits of infant rotavirus vaccination in England. Vaccine. 2014;32(29):3604–10.
Yung CF, Chan SP, Soh S, Tan A, Thoon KC. Intussusception and monovalent rotavirus vaccination in Singapore: self-controlled case series and risk-benefit study. J Pediatr. 2015;167(1):163–168.e1.
Ledent E, Lieftucht A, Buyse H, Sugiyama K, McKenna M, Holl K. Post-marketing benefit-risk assessment of rotavirus vaccination in Japan: a simulation and modelling analysis. Drug Saf. 2016;39(3):219–30.
Doubilet P, Begg CB, Weinstein MC, Braun P, McNeil BJ. Probabilistic sensitivity analysis using Monte Carlo simulation, a practical approach. Med Decis Making. 1985;5(2):157–77.
Melliez H, Boelle PY, Baron S, Mouton Y, Yazdanpanah Y. Morbidity and cost of rotavirus infections in France. Med Mal Infect. 2005;35(10):492–9.
Huet F, Chouchane M, Cremillieux C, Aubert M, Caulin E, Pothier P, et al. Prospective epidemiological study of rotavirus gastroenteritis in Europe (REVEAL study). Results in the French area of the study. Arch Pediatr. 2008;15(4):362–74.
Braeckman T, Van Herck K, Meyer N, Pircon JY, Soriano-Gabarro M, Heylen E, et al. Effectiveness of rotavirus vaccination in prevention of hospital admissions for rotavirus gastroenteritis among young children in Belgium: case-control study. BMJ. 2012;345:e4752.
Payne DC, Boom JA, Staat MA, Edwards KM, Szilagyi PG, Klein EJ, et al. Effectiveness of pentavalent and monovalent rotavirus vaccines in concurrent use among US children < 5 years of age, 2009-2011. Clin Infect Dis. 2013;57(1):13–20.
Ichihara MY, Rodrigues LC, Teles Santos CA, Teixeira Mda G, De Jesus SR, Alvim De Matos SM, et al. Effectiveness of rotavirus vaccine against hospitalized rotavirus diarrhea: a case-control study. Vaccine. 2014;32(23):2740–7.
INSEE. National Institute of Statistics and Economic Studies. Demography - live births - metropolitan France. 28 September 2017. https://www.insee.fr/en/statistiques/serie/000436391. Accessed 10 Oct 2017.
Martinot A, Cohen R, Denis F, Gaudelus J, Lery T, Le Danvic M, et al. Annual trends (2008–2011) in early childhood vaccination coverage for the French population: the Vaccinoscopie(R) study. Arch Pediatr. 2013;20(8):837–44.
Martinot A, Cohen R, Denis F, Gaudelus J, Lery T, Lepetit H, et al. Assessing early childhood vaccination coverage in France after 2013 vaccination schedule implementation. Arch Pediatr. 2014;21(12):1389–90.
Santé publique France. Rapport sur la politique vaccinale. January 2016. http://social-sante.gouv.fr/IMG/pdf/rapport_sur_la_politique_vaccinale_janvier_2016_.pdf. Accessed 12 Aug 2016.
INSERM. Centre d’épidémiologie sur les causes médicales de décès (CEPI-DC). http://www.cepidc.inserm.fr/inserm/html/index2.htm. Accessed 12 Aug 2016.
King CK, Glass R, Bresee JS, Duggan C. Managing acute gastroenteritis among children: oral rehydration, maintenance, and nutritional therapy. MMWR Recomm Rep. 2003;52(RR-16):1–16.
Diez-Domingo J, Martin IO, Sanz AB, Lopez AG, Martinez CC, Boronat CP, et al. Rotavirus gastroenteritis among children under five years of age in Valencia, Spain. Pediatr Infect Dis J. 2006;25(5):455–7.
Williams CJ, Lobanov A, Pebody RG. Estimated mortality and hospital admission due to rotavirus infection in the WHO European region. Epidemiol Infect. 2009;137(5):607–16.
Haut Conseil de la santé publique (HCSP), Vaccination des nourrissons contre les infections à rotavirus, Recommandations. 29 November 2013. http://www.hcsp.fr/explore.cgi/avisrapportsdomaine?clefr=404. Accessed 12 Aug 2016.
Standaert B, Strens D, Li X, Schecroun N, Raes M. The sustained rotavirus vaccination impact on nosocomial infection, duration of hospital stay, and age: the RotaBIS study (2005–2012). Infect Dis Ther. 2016;5(4):509–24.
Acknowledgements
The authors are in debt to Alfons Lieftucht (GSK), who contributed in developing the general benefit–risk framework for Rotarix that was adapted to the French setting in this manuscript. The authors would like to thank Vasile Coman (XPE Pharma and Science c/o GSK) for medical writing assistance and Adrian Kremer (XPE Pharma and Science c/o GSK) for editorial assistance and publication coordination on behalf of GSK.
Author information
Authors and Affiliations
Author notes
Edouard Ledent and Hugo Arlegui contributed equally.
Contributions
All named authors contributed to the design/acquisition of data or analysis and interpretation of data. They have provided substantial intellectual and scientific input into the development of this manuscript. All authors were involved in critically reviewing the content of the manuscript and its revision.
Corresponding author
Ethics declarations
Conflict of interest
Edouard Ledent, Hugo Arlegui, Hubert Buyse, Naveen Karkada, Gaëlle Nachbaur and Nicolas Praet are employed by the GSK group of companies. Hubert Buyse, Edouard Ledent, Gaëlle Nachbaur and Nicolas Praet also hold shares in the GSK group of companies. Peter Basile was an employee of the GSK group of companies at the time of the study and also held shares in the GSK group of companies. Hugo Arlegui is also a doctoral fellow whose research is financed by the Association Nationale pour la Recherche et la Technologie (ANRT) (Paris, France) and the GSK group of companies.
Funding
GlaxoSmithKline Biologicals SA was the funding source and was involved in all stages of study conduct and analysis. GlaxoSmithKline Biologicals SA also funded all costs associated with the development and the publishing of the present manuscript.
Trademarks
Rotarix is a trademark of the GSK group of companies. RotaTeq is a trademark of Merck & Co., Inc.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ledent, E., Arlegui, H., Buyse, H. et al. Benefit Versus Risk Assessment of Rotavirus Vaccination in France: A Simulation and Modeling Analysis. BioDrugs 32, 139–152 (2018). https://doi.org/10.1007/s40259-018-0273-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-018-0273-6