Abstract
Background
Tenecteplase (TNK-tPA) is a promising third-generation plasminogen activator, because of its greater fibrin specificity and longer half-life than alteplase. There is a paucity of studies on intravenous thrombolysis using TNK-tPA in developing countries. The present study has been undertaken to compare the efficacy and safety of TNK-tPA with alteplase.
Methods
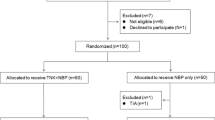
Two studies were conducted. Study I was an open-label, randomized study in which two doses of TNK-tPA (0.1 and 0.2 mg/kg) were compared. Study II was an open-label study in which TNK-tPA 0.2 mg/kg bolus was compared with historical controls. The primary endpoint for study I and study II was an improvement of ≥ 8 points or a score of 0 on the National Institutes of Health Stroke Scale (NIHSS) [major neurological improvement (MNI)] at 24 h. Secondary endpoints for both studies were neurological improvement as assessed using the NIHSS score, modified Rankin Scale (mRS) score and the Barthel Index (BI) on days 7, 30 and 90. Minimal disability was defined as an mRS score of 0 or 1 and good functional recovery as a BI score of 50–90. Safety was assessed by the proportion of patients having symptomatic intracranial hemorrhage (sICH) within 36 h and asymptomatic intracranial hemorrhage at 48 h after treatment.
Results
In study I, 20 patients received 0.1 mg/kg and 30 received 0.2 mg/kg TNK-tPA. There was no significant difference in MNI at 24 h between 0.1 and 0.2 mg/kg TNK-tPA doses. The patients given 0.2 mg/kg TNK-tPA had a significantly better 3-month outcome (minimal disability, p = 0.007). There was no sICH in study I. In study II, 62 patients (one lost to follow-up) received 0.2 mg/kg TNK-tPA. MNI was noted in ten patients (16.4%), 3-month minimal disability was noted in 37 patients (60.7%), and good functional recovery was seen in 33 patients (54.1%). sICH occurred in one patient, and four patients died. Pooled data of patients in study I and study II receiving 0.2 mg/kg TNK-tPA were compared with data from the historical National Institute of Neurological Disorders and Stroke (NINDS) trial. For comparison, the primary endpoint of the NINDS trial (improvement on NIHSS of ≥ 4 points or a score of 0 at 24 h) was taken. The primary endpoint though was not significantly different (58.2% vs. 47%, p = 0.08), but with TNK-tPA, greater neurological improvement, minimal disability (70.3 vs. 39%, p < 0.001) and good functional recovery (36.3 vs. 16%, p < 0.001) was noted at 3 months. There was a lower incidence of sICH (1.1 vs. 6.4%, p = 0.05) and lower 3-month mortality (5.5 vs. 17%, p = 0.01) noted with TNK-tPA compared with alteplase.
Conclusions
Intravenous TNK-tPA 0.2 mg/kg administered within 3 hours of symptom onset seems to be well tolerated and effective option in patients with acute ischemic stroke.
Trial Registration
Clinical Trials Registry—India, www.ctri.nic.in; unique identifiers: CTRI/2009/091/000251 and CTRI/2015/02/005556.

Similar content being viewed by others
References
Krishnamurthi RV, Feigin VL, Forouzanfar MH, Mensah GA, Connor M, Bennett DA, et al. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet Glob Health. 2013;1:e259–81. https://doi.org/10.1016/S2214-109X(13)70089-5.
Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, et al. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet. 2014;383:245–55.
Brown DL, Barsan WG, Lisabeth LD, Gallery ME, Morgenstern LB. Survey of emergency physicians about recombinant tissue plasminogen activator for acute ischemic stroke. Ann Emerg Med. 2005;46:56–60. https://doi.org/10.1016/j.annemergmed.2004.12.025.
Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384:1929–35. https://doi.org/10.1016/S0140-6736(14)60584-5.
Vandelli L, Marietta M, Gambini M, Cavazzuti M, Trenti T, Cenci MA, et al. Fibrinogen decrease after intravenous thrombolysis in ischemic stroke patients is a risk factor for intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2015;24:394–400. https://doi.org/10.1016/j.jstrokecerebrovasdis.2014.09.005.
Bhatia R, Hill MD, Shobha N, Menon B, Bal S, Kochar P, et al. Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke real-world experience and a call for action. Stroke. 2010;41:2254–8. https://doi.org/10.1161/STROKEAHA.110.592535.
Yepes M, Roussel BD, Ali C, Vivien D. Tissue-type plasminogen activator in the ischemic brain: more than a thrombolytic. Trends Neurosci. 2009;32:48–55. https://doi.org/10.1016/j.tins.2008.09.006.
Nagaraja D, Gururaj G, Girish N, Panda S, Roy AK, Sarma GRK, et al. Feasibility study of stroke surveillance: data from Bangalore, India. Indian J. Med. Res. 2009;130:396–403.
Durai Pandian J, Padma V, Vijaya P, Sylaja P, Murthy JM. Stroke and thrombolysis in developing countries. Int J Stroke. 2007;2:17–26. https://doi.org/10.1111/j.1747-4949.2007.00089.x.
Pandian JD, Sethi V, Dhillon R, Kaur R, Padala S, Chakravorty R, et al. Is intravenous thrombolysis feasible in a developing country? Cerebrovasc Dis. 2005;20:134–6. https://doi.org/10.1159/000086804.
Keyt BA, Paoni NF, Refino CJ, Berleau L, Nguyen H, Chow A, et al. A faster-acting and more potent form of tissue plasminogen activator. Proc Natl Acad Sci USA. 1994;91:3670–4.
Thomas GR, Thibodeaux H, Errett CJ, Badillo JM, Keyt BA, Refino CJ, et al. A long-half-life and fibrin-specific form of tissue plasminogen activator in rabbit models of embolic stroke and peripheral bleeding. Stroke. 1994;25:2072–8.
Haley EC, Lyden PD, Johnston KC, Hemmen TM. TNK in Stroke Investigators and others. A pilot dose-escalation safety study of tenecteplase in acute ischemic stroke. Stroke. 2005;36:607–12. https://doi.org/10.1161/01.STR.0000154872.73240.e9.
Haley EC, Thompson JL, Grotta JC, Lyden PD, Hemmen TG, Brown DL, For the tenecteplase in stroke Investigators, et al. Phase IIB/III trial of tenecteplase in acute ischemic stroke results of a prematurely terminated randomized clinical trial. Stroke. 2010;41:707–11. https://doi.org/10.1161/strokeaha.109.572040.
Parsons M, Spratt N, Bivard A, Campbell B, Chung K, Miteff F, et al. A randomized trial of tenecteplase versus alteplase for acute ischemic stroke. N Engl J Med. 2012;366:1099–107. https://doi.org/10.1056/NEJMoa1109842.
Huang X, Cheripelli BK, Lloyd SM, Kalladka D, Moreton FC, Siddiqui A, et al. Alteplase versus tenecteplase for thrombolysis after ischaemic stroke (ATTEST): a phase 2, randomised, open-label, blinded endpoint study. Lancet Neurol. 2015;14:368–76. https://doi.org/10.1016/S1474-4422(15)70017-7.
The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–7. https://doi.org/10.1056/nejm199512143332401.
Wahlgren N, Ahmed N, Dávalos A, Hacke W, Millán M, Muir K, for the SITS Investigators, et al. Thrombolysis with alteplase 3–4.5 h after acute ischaemic stroke (SITS-ISTR): an observational study. Lancet. 2008;372:1303–9. https://doi.org/10.1016/s0140-6736(08)61339-2.
Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, For the ECASS Investigators, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–29. https://doi.org/10.1056/nejmoa0804656.
Parsons M, Miteff F, Bateman G, Spratt N, Loiselle A, Attia J, et al. Acute ischemic stroke Imaging-guided tenecteplase treatment in an extended time window. Neurology. 2009;72:915–21. https://doi.org/10.1212/01.wnl.0000344168.05315.9d.
Acknowledgements
We would like to thank all the patients who participated in the study.
Funding
These studies were sponsored by Gennova Biopharmaceuticals, Ltd., Pune, India.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
Dr. Sunil Narayan was one of the members of the expert panels for the Neurology and Psychiatry Subject Expert Committee (SEC) duly constituted by the office of the Drug Controller General of India (DCGI). However, he was not a member of the Neurology and Psychiatry SEC which examined the data presentation and gave recommendations to the office of DCGI for the marketing authorization of tenecteplase. All other authors have declared that they have no conflicts of interest that might be relevant to the contents of this manuscript.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ramakrishnan, T.C.R., Kumaravelu, S., Narayan, S.K. et al. Efficacy and Safety of Intravenous Tenecteplase Bolus in Acute Ischemic Stroke: Results of Two Open-Label, Multicenter Trials. Am J Cardiovasc Drugs 18, 387–395 (2018). https://doi.org/10.1007/s40256-018-0284-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40256-018-0284-1