Abstract
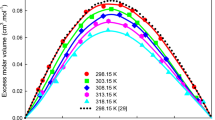
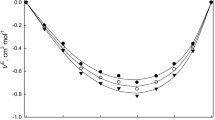
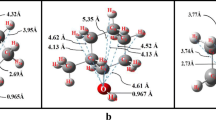
Densities of methyl nonanoate, n-dodecane, and their binary mixtures were investigated to provide the necessary data for their engineering applications as promising fuels and fuel additives. In the present work, densities were measured under atmospheric pressure at 293.15―463.15 K. The density data for the binary mixtures were fitted into a form of excess molar volume. The excess molar volumes were mostly positive, and the maximum value was obtained at molar fractions of n-dodecane between 0.5 and 0.6. Molecular simulations of specified systems were carried out by using four kinds of force fields, and the suitable force fields for describing the volume properties of the system were AMBER96 and OPLS-AA. The relative deviations for these two force fields between the simulated and the experimental data were well within ±4%, which meets the general engineering requirement.
Similar content being viewed by others
References
Guo P., Wang Q. Y., Li P. F., Environmental Science & Tech., 2018, 77(4), 67
Liu X. H., Jiang K., Modern Phys. Lett. B, 2018, 43(2), 57
Zhong W., Tamilselvan P., Wang Q., Energy, 2018, 67(5), 153
Geng P., Cao E., Tan Q., Renewable and Sustainable Energy Reviews, 2017, 71(2), 523
Shameer P. M., Ramesh K., Energy, 2017, 118(3), 1334
Motamedifar N., Shirneshan A., Fuel, 2018, 221(2), 233
Wang X., Wang X., Chen J., Fuel, 2016, 166(3), 553
Patra A., Samanta N., J. Chem. Phys., 2016, 145(16), 164
Abbaspour M., Akbarzadeh H., Salemi S., J. Molecular Liquids, 2018, 250(8), 26
Sun J., Qu J., Yan P. K., Natural, 2015, 23(1), 63
Sharma M., Wanchoo R. K., Toor A. P., Industrial & Engineering Chem. Research, 2012, 51(44), 14367
Haghbakhsh R., Raeissi S., J. Chem. Thermodynamics, 2018, 98(23), 533
Cui S., Khomami B., J. Phys. Chem. B, 2014, 118(36), 10750
Sobrino M., Eduardo I., J. Chem. Thermodynamics, 2016, 98(14), 231
Tay W. J., Trusler J. P. M., J. Chem. Thermodynamics, 2018, 35(9), 156
Zarei H., Mohamadkhani R., Fluid Phase Equilibria, 2016, 409(3), 19
Mcatee Z. P., Heitz M. P., J. Chem. Thermodynamics, 2016, 93(16), 34
Rao P. V., Venkatramana L., Gowrisankar M., J. Chem. Thermodynamics, 2016, 94(7), 186
Aniya V. K., Muktham R. K., Alka K., Fuel, 2015, 161(15), 137
Kawakami T., Shigemoto I., Matubayasi N., J. Chem. Phys., 2018, 148(21), 214903
Bharadwaj V. S., Eagan N. M., Wang N. M., Chemphyschem, 2015, 16(13), 2810
Belashchenko, Russian J. Phys. Chem. A, 2016, 90(9), 1707
Caro M. A., Laurila T., Lopez-Acevedo O., J. Chem. Phys., 2016, 145(24), 244504
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Natural Science Foundation of China(No.51506140).
Rights and permissions
About this article
Cite this article
Zang, D., Zhao, G., Liu, X. et al. Experimental and Molecular Simulation of Volumetric Properties of Methyl Nonanoate, n-Dodecane, and Their Binary Mixtures. Chem. Res. Chin. Univ. 35, 299–303 (2019). https://doi.org/10.1007/s40242-019-8249-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40242-019-8249-8