Abstract
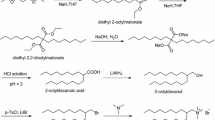
Novel Brönsted acid-surfactants with different alkyl chains were synthesized via a two-step process, and their surface properties were studied. The critical micelle concentration(cmc), surface tension at the cmc(γcmc), and ability of these compounds to lower the surface tension by 0.02 N/m(C20 and pC20) were investigated at 25 and 40 °C. The molecular architecture of the compounds strongly influenced these physicochemical parameters. The ability of these compounds to lower surface tension was found to be good. Etherification in microemulsions formed by these surfactants as well as dodecylbenzenesulfonic acid(DBSA) was performed; surfactants 3a and 3b were found to be much more efficient than DBSA.
Similar content being viewed by others
References
Domínguez R., Rodríguez A., Maestre A., Robina I., Moyá M. L., J. Colloid Inter. Sci., 2012, 386(1), 228
Mouraa E. F., Netoa A. O. W., Dantasa T. N., Júniora H. S., Gurgel A., Colloid. Surfaces A 2009, 340, 199
Chen J., Qiao M., Gao N., Ran Q., Wu S., Qi S., Colloid. Surface A 2017, 522, 593
Wang X., Li R., Li Z., Liu J., J. Colloid Inter. Sci., 2017, 505, 847
Manabe K., Limura S., Sun X.M., Kobayashi S., J. Am. Chem. Soc., 2002, 124, 11971
Limura S., Manabe K., Kobayashi S., Org. Lett. 2003, 5, 101
Jang H., Lee H., Colloid. Surfaces A 2018, 538, 574
Menger F. M., Elringtn A. R., J. Am. Chem. Soc., 1991, 113, 9621
Yin J. C., Chen Y. K., Jiang J. Z., Cui Z. G., Chem. J. Chinese Universities, 2017, 38(9), 1645
Liu X., Xing X., Gao Z., Colloid. Surfaces A 2014, 457, 374
Dong D., Ouyang Y., Yu H., Liu Q., Liu J., Wang M., Zhu J., J. Org. Chem., 2005, 70, 4535
Fernando Silva O., de Rossia Rita H., Mariano Correa N., RSC Adv. 2015, 5, 34878
Jing L., Li X., Han Y., Chu Y., Colloid. Surface A 2008, 326, 37
Han Y., Chu Y., J. Mol. Catal. A: Chem., 2005, 273, 232
Song K., Chu Y., Dong L., Song J., Wang D., J. Mol. Catal. A: Chem., 2008, 282, 144
Malferrari D., Armenise N., Decessari S., Galletti P., Tagliavini E., ACS Sustain Chem. Eng., 2015, 3, 1579
Wang X., Yan F., Li Z., Zhang L., Zhao S., An J., Yu J., Colloid. Surfaces A 2007, 302, 532
You A., Cao Y., Cao G., Chem. Res. Chinese Universities 2017, 33(4), 525
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supported by the National Natural Science Foundation of China(No. 51373067).
Rights and permissions
About this article
Cite this article
Yang, M., Wei, Z., Duan, H. et al. Surface Properties and Etherification in Microemulsion Systems of Novel Brönsted Acid Surfactants. Chem. Res. Chin. Univ. 34, 440–443 (2018). https://doi.org/10.1007/s40242-018-8046-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40242-018-8046-9