Abstract
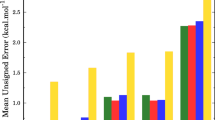
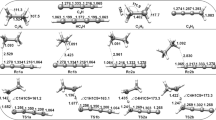
Ab initio study of the reactions of n-heptyl radicals(1-C7H15, 2-C7H15, 3-C7H15, and 4-C7H15) with methanol was conducted over the temperature range of 300–1500 K. Transition states for the reaction channels producing C7H15OH, CH3, C7H15OCH3, H, C7H16, CH2OH and CH3O were identified and the geometries of all stationary points were calculated at BB1K/MG3S level of theory. The potential barrier heights of the corresponding transition states were predicted by the CBS-QB3//BB1K and G4//BB1K methods, indicating that the eight H-abstraction channels are more kinetically favorable than the channels where OH transfers from CH3OH to C7H15 and where the C7H15OCH3+H products are given. The rate constants of H-abstraction channels were calculated with TST and TST/Eck. Both the forward and reverse rate constants have positive temperature dependence and the tunneling effect is only important at the temperature lower than 700 K. For the reactions of H-atom abstraction from methyl in CH3OH by n-heptyl, a reverse and the corresponding forward rate constant are roughly equal. For the reactions of H-atom abstraction from OH in CH3OH by n-heptyl, a reverse rate constant is larger by several orders of magnitude than the corresponding forward one.
Similar content being viewed by others
References
Sadeghinezhad E., Kazi S. N., Sadeghinejad F., Badarudin A., Me-hrali M., Sadri R., Safaei M. R., Renew. Sust. Energ. Rev., 2014 30(30), 29
Balki M. K., Sayin C., Canakci M., Fuel, 2014 115(4), 901
Hsieh W. D., Chen R. H., Wu T. L., Lin T. H., Atmos. Environ., 2002 36(3), 403
Agarwal A. K., Karare H., Dhar A., Fuel. Process. Technol., 2014 121(5), 16
Tran L. S., Glaude P. A., Fournet R., Battin-Leclerc F., Energy Fuels, 2013 27(4), 2226
Lee P. F., Matsui H., Xu D. W., Wang N. S., J. Phys. Chem. A, 2013 117(3), 525
Held T. J., Dryer F. L., Int. J. Chem. Kinet., 1998 30(11), 805
Curran H. J., Gaffuri P., Pitz W. J., Westbrook C. K., Combus. Flame, 1998, 114(1/2), 149
Zheng Z. L., Yao M. F., Fuel, 2006, 85(17/18), 2605
Chen C. X., Song, J. O., Song C. L., Lv G., Int. J. Chem. Kinet., 2015 47(12), 764
Alecu I. M., Truhlar D. G., J. Phys. Chem. A, 2011 115(51), 14599
Shi J., Ran J. Y., Qin C. L., Qi W. J., Zhang L., Comput. Theor. Chem., 2015 1074, 73
Park J., Xu Z. F., Xu K., Lin M. C., P. Combust. Inst., 2013 34(1), 473
Zhao Y., Lynch B. J., Truhlar D. G., J. Phys. Chem. A, 2004 108(14), 2715
Lynch B. J., Zhao Y., Truhlar D. G., J. Phys. Chem. A, 2003 107(9), 1384
Becke A. D., J. Chem. Phys., 1992 96(3), 2155
Becke A. D., J. Chem. Phys., 1992 97(12), 9173
Becke A. D., J. Chem. Phys., 1993 98(7), 5648
Katsikadakos D., Hardalupas Y., Taylor A. M., Hunt P. A., Phys. Chem. Chem. Phys., 2012 14(27), 9615
Chen C. X., Song J. O., Song C. L., Lv G., Li Z. J., Mol. Phys., 2015 114(2), 315
Chen C. X., Song J. O., Song C. L., Lv G., Li Z. J., Comput. Theor. Chem., 2016 1075(2), 63
Ma P., Song J. O., Song C. L., Lv G., Chen C. X., Yang C. W., Chem. J. Chinese Universities, 2015 36(1), 149
Coote M. L., J. Phys. Chem. A, 2004 108(17), 3865
Alecu I. M., Zheng J. J., Zhao Y., Truhlar D. G., J. Chem. Theory Comput., 2010 6(9), 2872
Fukui K., J. Phys. Chem., 1970 74(23), 4161
Curtiss L. A., Redfen P. C., Raghavachari K., J. Chem. Phys., 2007 126(8), 084108
Montgomery Jr. J. A., Frisch M. J., Ochterski J. W., Petersson G. A., J. Chem. Phys., 1999 110(6), 2822
Purvis III G. D., Bartlett R. J., J. Chem. Phys., 1982 76(4), 1910
Pople J. A., Head-Gordon M., Raghavachari K., J. Chem. Phys., 1987 87(10), 5968
Kendall R. A., Dunning Jr T. H., Harrison R. J., J. Chem. Phys., 1992 96(9), 6796
Frisch M. J., Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Scalmani G., Barone V., Mennucci B., Peters-son G. A., Nakatsuji H., Caricato M., Li X., Hratchian H. P., Izmay-lov A. F., Bloino J., Zheng G., Sonnenberg J. L., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Vreven T., Montgomery Jr. J. A., Peralta J. E., Ogliaro F., Bearpark M. J., Heyd J., Brothers E. N., Kudin K. N., Staroverov V. N., Kobayashi R., Normand J., Raghavachari K., Ren-dell A. P., Burant J. C., Iyengar S. S., Tomasi J., Cossi M., Rega N., Millam N. J., Klene M., Knox J. E., Cross J. B., Bakken V., Adamo C., Jaramillo J., Gomperts R., Stratmann R. E., Yazyev O., Austin A. J., Cammi R., Pomelli C., Ochterski J. W., Martin R. L., Morokuma K., Zakrzewski V. G., Voth G. A., Salvador P., Dannenberg J. J., Dapprich S., Daniels A. D., Farkas O., Foresman J. B., Ortiz J. V., Cioslowski J., Fox D. J., Gaussian 09, Gaussian, Inc., Wallingford, CT,2009
Eyring H., Chem. Rev., 1935 17(1), 65
Barker J. R., Nguyen T. L., Stanton J. F., Aieta C., Ceotto M., Gabas F., Kumar T. J. D., Li C. G. L., Lohr L. L., Maranzana A., Ortiz N. F., Preses J. M., Simmie J. M., Sonk J. A., Stimac P. J., MultiWell-2017 Software Suite, Ann Arbor, Michigan, 2017
Eckart C., Phys. Rev., 1930 35(11), 1303
Ayala P. Y., Schlegel H. B., J. Chem. Phys., 1998 108(6), 2314
NIST Computational Chemistry Comparison and Benchmark Data-base. Release 18(October 2016). https://doi.org/cccbdb.nist.gov/
Lynch B. J., Truhlar D. G., J. Phys. Chem. A, 2001 105(105), 2936
Hammond G.S., J. Am. Chem. Soc., 1955 77(2), 334
Lee T. J., Taylor P. R., Int. J. Quantum Chem., 1989, 36(23 S), 199
Orlov M. Y., Chernova E. M., Turovtsev V. V., Orlov Y. D., Russ. Chem. Bull., 2014 63(12), 2620
Liu J. Y., Li Z. S., Wu J. Y., Wei Z. G., Zhang G., Sun C. C., J. Chem. Phys., 2003 119(14), 7214
Canneaux S., Bohr F., Henon E., J. Comput. Chem., 2014 35(1), 82
Jodkowski J. T., Rayez M. T., Rayez J. C., Berces T., Dobe S., J. Phys. Chem. A, 1999 103(19), 3750
Menrad H., Nierhauve B., SAE Technical Paper, 1983, 831686
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by the National Natural Science Foundation of China(No.51576139).
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Zhao, Z., Song, J., Su, B. et al. Theoretical Study on the Kinetics for the Reactions of Heptyl Radicals with Methanol. Chem. Res. Chin. Univ. 34, 786–791 (2018). https://doi.org/10.1007/s40242-018-8026-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40242-018-8026-0