Abstract
Objective
Oxidative stress resulting from chronic hyperglycemia induced many complications in diabetes and led to disorders and dysfunctions in different organs. This study aimed to evaluate the hepatoprotective rate of cress seeds (CS) or Lepidium sativum seeds in the diet on lowering hyperglycemia and oxidative stress damaging.
Methods
Diabetes was induced by a single intraperitoneal injection of 60 mg/kg of streptozotocin (STZ). Forty-eight male rats were randomly divided into six groups : (D-0) and (ND-0) diabetic, and non-diabetic groups were fed with a normal diet, (ND-CS2) and (ND-CS5) non-diabetic groups were fed with diet containing 2 % and 5 % of cress seeds respectively, (D-CS2) and (D-CS5) diabetic groups were fed with diet containing 2 % and 5 % of cress seeds respectively. After 28 days of treatment, biochemical, histological, and oxidative parameters were determined. Hepatic and pancreatic histological sections were developed.
Results
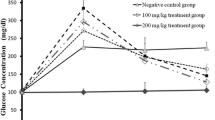
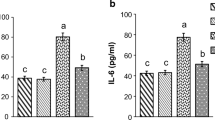
STZ-injection caused hyperglycemia accompanied by a disturbance in biochemical parameters and intensified oxidative stress status compared to the (ND-0) group. Hepatic and pancreatic histological sections of diabetic rats showed a disrupted architecture. However, the cress seeds-diet revealed a significant decrease of hyperglycemia and a reduction of the intensity of oxidative stress induced by diabetes compared to the (D-0) group, remarked by a decreased level of Malondialdehyde (MDA) and high levels of glutathione (GSH) and the antioxidant enzymes, led to the decrease of the majority of parameters principally hepatic and lipid profile with histological regeneration.
Conclusions
Cress seeds supplementation confirmed their potential anti-diabetic and antioxidant activities with higher efficacy of 5 % dose than the lower dose of 2 %. Therefore, 5 % of cress seeds administration seems to be the excellent rate recommended in controlling diabetes and its complications.







Similar content being viewed by others
References
El Barky AR, Ezz AA, Alm-Eldeen H, Hussein AAE, Hafez SA, Mohamed YA. Can stem cells ameliorate the pancreatic damage induced by streptozotocin in rats? Can J Diabetes. 2017;42(1):61–70.
Ghosh S, Chowdhury S, Sarkar P, Sil PC. Ameliorative role of ferulic acid against diabetes-associated oxidative stress-induced spleen damage. Food Chem Toxicol. 2018;118:272–86.
Chauhan K, Sharma S, Agarwal N, Chauhan S, Chauhan B. A study on potential hypoglycemic and hypolipidemic effects of Lepidium Sativum (Garden Cress) in Alloxan induced diabetic rats. Am J Pharm Tech Res. 2012;2(3):522–35.
Aouacheri O, Saka S, Krim M, Messaadia A, Maidi I. The investigation of the oxidative stress-related parameters in type 2 diabetes mellitus. Can J Diabetes. 2015;39(1):44–9.
Attia ES, Amer AH, Hassanein MA. The hypoglycemic and antioxidant activities of garden cress (Lepidium sativum L.) seed on alloxan-induced diabetic male rats. Nat Prod Res. 2017;33(6):901–5.
Paschou SA, Papadopoulou-Marketou N, Chrousos GP, Kanaka-Gantenbein C. On type 1 diabetes mellitus pathogenesis. Endocrine Connect. 2018;7(1):R38-46.
Ghayati Z. Antioxydants and diabetes type 2 (Doctoral dissertation). 2019. URL: http://hdl.handle.net/123456789/17389.
Malar MJ, Vanmathi JS, Chairman K. Antidiabetic activity of different parts of the plant Lepidium sativum Linn. Asian J App Sci Technol. 2017;1(9):135–41.
Yan LJ. Pathogenesis of chronic hyperglycemia: from reductive stress to oxidative stress. J Diabetes Res. 2014;2014:1–11.
Luo X, Wu J, Jing S, Yan LJ. Hyperglycemic stress and carbon stress in diabetic glucotoxicity. Aging Dis. 2016;7(1):90.
Robson R, Kundur AR, Singh I. Oxidative stress biomarkers in type 2 diabetes mellitus for assessment of cardiovascular disease risk. Diabetes Metab Syndr Clin Res Rev. 2018;12(3):455–62.
Laura A, Klibet F, Bourogaa E, Benamara A, Boumendjel A, Chefrour A, Messiah M. Potential antioxidant properties and hepatoprotective effects of Juniperus phoenicea berries against CCl4 induced hepatic damage in rats. Asian Pac J Trop Med. 2017;10(3):263-9.
Kamkar MMA, Ahmad R, Alsmadi O, Behbehani K. Insight into the impact of diabetes mellitus on the increased risk of hepatocellular carcinoma: mini-review. J Diabetes Metab Disord. 2014;13(1):57.
Karigidi KO, Akintimehin ES, Omoboyowa DA, Adetuyi FO, Olaiya CO. Effect of Curculigo pilosa supplemented diet on blood sugar, lipid metabolism, hepatic oxidative stress and carbohydrate metabolism enzymes in streptozotocin-induced diabetic rats. J Diabetes Metab Disord. 2020;2020:1–12.
Tran TQ, Hsu YM, Huang YC, Chen CJ, Lin WD, Lin YJ, Chen SY. Integrated analysis of gene modulation profile identifies pathogenic factors and pathways in the liver of diabetic mice. J Diabetes Metab Disord. 2019;8(2):471–85.
Mohamed J, Nafizah AN, Zariyantey AH, Budin S. Mechanisms of diabetes-induced liver damage: the role of oxidative stress and inflammation. Sultan Qaboos Univ Med J. 2016;16(2):e132.
Mishra N, Mohammed A, Rizvi SI. Efficacy of Lepidium Sativum to act as an anti-diabetic agent. Prog Health Sci. 2017;7(1):44–53.
Desai SS, Walvekar MV, Shaikh NH. Cytoprotective effects of Lepidium sativum seed extract on liver and pancreas of HFD/STZ induced type 2 diabetic mice. Inter J Pharm Phytochem Res. 2017;9(4):502–7.
Kumar V, Tomar V, Ranade SA, Yadav HK, Srivastava M. Phytochemical, antioxidant investigations and fatty acid composition of Lepidium sativum seeds. J Environ Biol. 2020;41(1):59–65.
Raish M, Ahmad A, Alkharfy KM, Ahamad SR, Mohsin K, Al-Jenoobi F, Ansari MA. Hepatoprotective activity of Lepidium sativum seeds against D-galactosamine/ lipopolysaccharide-induced hepatotoxicity in animal model. BMC Complement Altern Med. 2016;16(1):501.
Al-Sheddi ES, Farshori NN, Al-Oqail MM, Musarrat J, Al-Khedhairy AA, Siddiqui MA. Protective effect of Lepidium sativum seed extract against hydrogen peroxide-induced cytotoxicity and oxidative stress in human liver cells (HepG2). Pharm Biol. 2016;54(2):314–21.
Wu J, Yan LJ. Streptozotocin-induced type 1 diabetes in rodents as a model for studying mitochondrial mechanisms of diabetic β cell glucotoxicity. Diabetes Metab Syndr Obes Target Ther. 2015;8:181.
Weckbecker G, Cory JG. Ribonucleotide reductase activity and growth of glutathione-depended mouse leukaemia L1210 cells in vitro. Cancer Lett. 1988;40:257–64.
Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8.
Flohé L, Günzler WA. Assays of glutathione peroxidase. Methods Enzym. 1984;105:114–20.
Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249(22):7130-9.
Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–6.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1–2):248–54.
Saka S, Aouacheri O. The investigation of the oxidative stress-related parameters in high doses methotrexate-induced albino Wistar rats. J Bioequiv Availab. 2017;9:372-6.
Houlot R. Techniques d’histopathologie et de cytopathologie. Paris: Editions Maloine; 1984.
Al-Ishaq RK, Abotaleb M, Kubatka P, Kajo K, Büsselberg D. Flavonoids and their anti-diabetic effects: cellular mechanisms and effects to improve blood sugar levels. Biomolecules. 2019;9(9):430.
Chowdhury S, Ghosh S, Rashid K, Sil PC. Deciphering the role of ferulic acid against streptozotocin-induced cellular stress in the cardiac tissue of diabetic rats. Food Chem Toxicol. 2016a;97:187–98.
Tebboub I, Kechrid Z. Effect of Curcuma on zinc, lipid profile and antioxidants levels in blood and tissue of streptozotocin-induced diabetic rats fed zinc deficiency diet. Arch Physiol Biochem. 2019;2019:1–8.
Kim JD, Kang SM, Seo BI, Choi HY, Choi HS, Ku SK. Anti-diabetic activity of SMK001, a polyherbal formula in streptozotocin-induced diabetic rats: therapeutic study. Biol Pharm Bull. 2006;29(3):477–82.
Zafar M, Naqvi SNUH. Effects of STZ-induced diabetes on the relative weights of kidney, liver and pancreas in albino rats: a comparative study. Int J Morphol. 2010;28(1):135–42.
Wang F, Li H, Zhao H, Zhang Y, Qiu P, Li J, Wang S. Antidiabetic activity and chemical composition of Sanbai melon seed oil. Evid Based Complement Alternat Med. 2018;2018:1–14.
Lanjhiyana S, Garabadu D, Ahirwar D, Bigoniya P, Rana AC, Patra KC, Karuppaih M. Antidiabetic activity of methanolic extract of stem bark of Elaeodendron glaucum Pers. in alloxanized rat model. Adv Appl Sci Res. 2011;2(1):47–62.
Pitchai D, Manikkam R. Hypolipidemic, hepato-protective and renal damage recovering effects of catechin isolated from the methanolic extract of Cassia fistula stem bark on Streptozotocin-induced diabetic Wistar rats: a biochemical and morphological analysis. Med Chem Res. 2012;21(12):4535–41.
Al-khazraji SM. Biopharmacological studies of the aqueous extract of Lepidium sativum seeds in alloxan-induced diabetes in rats. Iraqi J Vet Med. 2012;36(2):158–63.
Shukla AK, Bigoniya P, Soni P. Hypolipidemic activity of Lepidium sativum Linn. seed in rats. IOSR J Pharm Biol Sci. 2015;10(4):13–22.
Halaby MS, Farag MH, Mahmoud SA. Protective and curative effect of garden cress seeds on acute renal failure in male albino rats. Middle East J Appl Sci. 2015;5(2):573–86.
Rajasekar R, Manokaran K, Rajasekaran N, Duraisamy G, Kanakasabapathi D. Effect of Alpinia calcarata on glucose uptake in diabetic rats-an in vitro and in vivo model. J Diabetes Metab Disord. 2014;13(1):33.
Hanhineva K, Törrönen R, Bondia-Pons I, Pekkinen J, Kolehmainen M, Mykkänen H, Poutanen K. Impact of dietary polyphenols on carbohydrate metabolism. Int J Mol Sci. 2010;11(4):1365–402.
Qusti S, El Rabey HA, Balashram SA. The hypoglycemic and antioxidant activity of cress seed and cinnamon on streptozotocin induced diabetes in male rats. Evid BasedComplement Alternat Med. 2016;2016:1–15.
Eddouks M, Maghrani M, Zeggwagh NA, Michel JB. Study of the hypoglycaemic activity of Lepidium sativum L. aqueous extract in normal and diabetic rats. J Ethnopharmacol. 2005;97(2):391–5.
Prajapati VD, Maheriya PM, Jani GK, Patil PD, Patel BN. Lepidium sativum Linn.: a current addition to the family of mucilage and its applications. Int J Biol Macromol. 2014;65:72–80.
Radwan HM, El-Missiry MM, Al-Said WM, Ismail A, Abdel Shafeek KA, Seif-El-Nasr MM. Investigation of the glucosinolates of Lepidium sativum growing in Egypt and their biological activity. Res J Med Med Sci. 2007;2(2):127–32.
Shukla A, Bigoniya P, Srivastava B. Hypoglycemic activity of Lepidium sativum Linn seed total alkaloid on alloxan-induced diabetic rats. Res J Med Plant. 2012;6(8):587–96.
Vinayagam R, Xu B. Antidiabetic properties of dietary flavonoids: a cellular mechanism review. Nutr Metab. 2015;12(1):1–20.
Yao Y, Zang Y, Qu J, Tang M, Zhang T. The toxicity of metallic nanoparticles on liver: the subcellular damages, mechanisms, and outcomes. Int J Nanomed. 2019;14:8787–804.
Vozarova B, Stefan N, Lindsay RS, Saremi A, Pratley RE, Bogardus C, Tataranni PA. High alanine aminotransferase is associated with decreased hepatic insulin sensitivity and predicts the development of type 2 diabetes. Diabetes. 2002;51(6):1889–95.
Al-Khazraji SM. Biopharmacological studies of the aqueous extract of Lepidium sativum seeds in alloxan-induced diabetes in rats. Iraqi J Vet Med. 2012;36(2):158–63.
Achi NK, Ohaeri OC, Ijeh II, Eleazu C. Modulation of the lipid profile and insulin levels of streptozotocin-induced diabetic rats by ethanol extract of Cnidoscolus aconitifolius leaves and some fractions: Effect on the oral glucose tolerance of normoglycemic rats. Biomed Pharmacother. 2017;86:562-9.
Laufs U, Parhofer KG, Ginsberg HN, Hegele RA. Clinical review on triglycerides. Europ Heart J. 2019;0:1–14.
Alharbi FK, Sobhy HM. Influence of dietary supplementation of garden cress (Lepidium sativum L.) on histopathology and serum biochemistry in Diabetic Rats. Egyptian J Chem Env Health. 2017;3(1):1–19.
El Barky AR, Hussein SA. Alm-Eldeen AA, Hafez YA, Mohamed TM. Anti-diabetic activity of Holothuria thomasi saponin. Biomed Pharma. 2016;84:1472-87.
Amawi K, Aljamal A. Effect of Lepidium sativum on lipid profiles and blood glucose in rats. J Phys Pharm Adv. 2012;2(8):277–81.
Ayepola OR, Brooks NL, Oguntibeju OO. Oxidative stress and diabetic complications: the role of antioxidant vitamins and flavonoids. 2014; [Online] Available at: https://doi.org/10.5772/57282.
Kawahito S, Kitahata H, Oshita S. Problems associated with glucose toxicity: role of hyperglycemia-induced oxidative stress. World J Gastroenterol. 2009;15(33):4137–42.
Rains JL, Jain SK. Oxidative stress, insulin signaling, and diabetes. Free Radical Biol Med. 2011;50(5):567–75.
Asmat U, Abad K, Ismail K. Diabetes mellitus and oxidative stress-A concise review. Saudi Pharm J. 2016;24(5):547–53.
Adeyemi DO, Ukwenya VO, Obuotor EM, Adewole SO. Anti-hepatotoxic activities of Hibiscus sabdariffa L. in animal model of streptozotocin diabetes-induced liver damage. BMC Compl Altern Med. 2014;14(1):277.
Mendes-Braz M, Martins JO. Diabetes mellitus and liver surgery: the effect of diabetes on oxidative stress and inflammation. Mediat Inflam. 2018;2018:1–11.
Palma HE, Wolkmer P, Gallio M, Corrêa MM, Schmatz R, Thomé GR, Pereira LB, Castro VS, Pereira AB, Bueno A, de Oliveira LS, Rosolen D, Mann TR, de Cecco BS, Graça DL, Lopes ST, Mazzanti CM. Oxidative stress parameters in blood, liver, and kidney of diabetic rats treated with curcumin and/or insulin. Mol Cell Biochem. 2014;386(1–2):199–210.
Ghosh S, Bhattacharyya S, Rashid K, Sil PC. Curcumin protects rat liver from streptozotocin-induced diabetic pathophysiology by counteracting reactive oxygen species and inhibiting the activation of p53 and MAPKs mediated stress response pathways. Toxicol Rep. 2015;2:365–76.
Sekou O, Boumendjel M, Taibi F, Boumendjel A, Messarah M. Mitigating effects of antioxidant properties of Artemisia herba alba aqueous extract on hyperlipidemia and oxidative damage in alloxan-induced diabetic rats. Arch Physiol Biochem. 2019;125(2):163–73.
Patel H, Chen J, Das KC, Kavdia M. Hyperglycemia induces differential change in oxidative stress at gene expression and functional levels in HUVEC and HMVEC. Cardiovasc Dialectal. 2013;12(1):142–6.
Prabakaran D, Ashokkumar N. Protective effect of esculetin on hyperglycemia-mediated oxidative damage in the hepatic and renal tissues of experimental diabetic rats. Biochimie. 2013;95(2):366–73.
El-Zawahry BH, El-Shawwa MM, Hikal FS. Effect of Lepidium sativum on blood levels of apelin and some metabolic and oxidative parameters in obese male rats. Al-Azhar Med J. 2017;46(3):723–38.
Madić V, Petrović A, Jušković M, Jugović D, Djordjević L, Stojanović G, Vasiljević P. Polyherbal mixture ameliorates hyperglycemia, hyperlipidemia and histopathological changes of pancreas, kidney and liver in a rat model of type 1 diabetes. J Ethnopharmacol. 2020;265:113210.
Acknowledgements
This work is supported by research project (№: D01N01UN230120190004) under the leadership of Professor S. Saka, and funded by the Ministry of Higher Education, Algeria. We would like to thank members of the Algiers Pasteur Institute for providing rats.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of interest for the authorship and/or publication of this article.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Doghmane, A., Aouacheri, O., Laouaichia, R. et al. The investigation of the efficacy ratio of cress seeds supplementation to moderate hyperglycemia and hepatotoxicity in streptozotocin‐induced diabetic rats. J Diabetes Metab Disord 20, 447–459 (2021). https://doi.org/10.1007/s40200-021-00764-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-021-00764-9