Abstract
Introduction
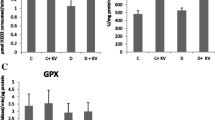
Reactive oxygen species (ROS) and lipid peroxidation (LPO) levels may increase in diabetic state and lead to oxidative stress, which plays a critical role in the progression of diabetes. There are various sources of ROS, including cytochrome P450 monooxygenases (CYP450s), which may be modulated in terms of their activities and expressions under diabetic conditions. This study is aimed to investigate the effects of streptozotocin-induced diabetes and insulin treatment on hepatic cytochrome P450 1A1 (CYP1A1) and cytochrome P450 2E1 (CYP2E1) activities and LPO levels. Methods: CYP1A1 and CYP2E1 activities were measured with ethoxyresorufin O-deethylase and p-nitrophenol hydroxylase activities, respectively. LPO levels were then corroborated via thiobarbituric acid reactive substances. Results: In diabetic rats, a marked 2.1- and 2.4-fold increase in hepatic CYP1A1 activity and 1.8- and 1.6-fold increase in hepatic CYP2E1 activity were observed compared to controls and insulin-treated diabetic rats, respectively. Hepatic LPO levels in diabetic rats did not significantly change compared to controls. However, in insulin-treated diabetic rats, LPO levels are 0.92- and 0.89-fold remarkably decrease compared to controls and diabetics, respectively. Conclusion: The present study suggests that insulin might have a useful role in the modulation of CYP1A1 and CYP2E1 activities as well as LPO levels in the liver of diabetic rats.


Similar content being viewed by others
Data svailability
Data are accessible upon request from the authors.
Abbreviations
- CYP1A1:
-
cytochrome P450 1A1
- CYP2E1:
-
cytochrome P450 2E1
- CYP450:
-
cytochrome P450 monooxygenase
- EROD:
-
7-ethoxyresorufinO-deethylase
- Fe++ :
-
Iron(II)
- KCI:
-
potassiumchloride
- LPO:
-
lipid peroxidation
- M:
-
molarity
- MDA:
-
malondialdehyde
- MgCI2 :
-
magnesiumchloride
- mins:
-
minutes
- mM:
-
millimolar
- N:
-
normality
- NADP+ :
-
nicotinamideadenine dinucleotide phosphate
- NaOH:
-
sodiumhydroxide
- nm:
-
nanometer
- ºC:
-
degreesCelsius
- pNP:
-
p-nitrophenol
- ROS:
-
reactive oxygen species
- TBARS:
-
thiobarbituricacid reactive substances
- Tris.HCl:
-
trishydrochloride
- U/mL:
-
unitspermillilitre
- v/v:
-
volume/volume
- w/v:
-
weight/volume
- µL:
-
microliter
References
Raza H, Ahmed I, John A, Sharma AK. Modulation of xenobiotic metabolism and oxidative stress in chronic streptozotocin-induced diabetic rats fed with Momordica charantia fruit extract. J Biochem Mol Toxicol. 2000;14(3):131–9.
Raza H, Prabu SK, Robin MA, Avadhani NG. Elevated mitochondrial cytochrome P450 2E1 and glutathione S-transferase A4-4 in streptozotocin-induced diabetic rats: tissue-specific variations and roles in oxidative stress. Diabetes. 2004;53(1):185–94.
König M, Bulik S, Holzhütter H-G. Quantifying the contribution of the liver to glucose homeostasis: a detailed kinetic model of human hepatic glucose metabolism. PLoS Comput Biol. 2012;8(6):e1002577.
Niedowicz DM, Daleke DL. The role of oxidative stress in diabetic complications. Cell Biochem Biophys. 2005;43(2):289–330. https://doi.org/10.1385/cbb:43:2:289.
Cederbaum AI. Role of Cytochrome P450 and oxidative stress in alcohol-induced liver injury. React Oxygen Species. 2017;4(11):303–19.
Memişoğulları R. Diyabette serbest radikallerin rolü ve antioksidanların etkisi düzce. Tıp Fakültesi Dergisi. 2005;3:30–9.
Dib M, Garrel C, Favier A, Robin V, Desnuelle C. Can malondialdehyde be used as a biological marker of progression in neurodegenerative disease? J Neurol. 2002;249(4):367–74. https://doi.org/10.1007/s004150200025.
Hall ED, Wang JA, Bosken JM, Singh IN. Lipid peroxidation in brain or spinal cord mitochondria after injury. J Bioenerg Biomembr. 2016;48(2):169–74.
Manikandan P, Nagini S. Cytochrome P450 structure, function and clinical significance: a review. Curr Drug Targets. 2018;19(1):38–54.
Guengerich FP. The 1992 Bernard B. Brodie Award Lecture. Bioactivation and detoxication of toxic and carcinogenic chemicals. Drug Metab Dispos Biol Fate Chem. 1993;21(1):1–6.
Puga A, Nebert DW, McKinnon RA, Menon AG. Genetic polymorphisms in human drug-metabolizing enzymes: potential uses of reverse genetics to identify genes of toxicological relevance. Crit Rev Toxicol. 1997;27(2):199–222. https://doi.org/10.3109/10408449709021619.
Stiborova M, Martinek V, Rydlova H, Koblas T, Hodek P. Expression of cytochrome P450 1A1 and its contribution to oxidation of a potential human carcinogen 1-phenylazo-2-naphthol (Sudan I) in human livers. Cancer Lett. 2005;220(2):145–54. https://doi.org/10.1016/j.canlet.2004.07.036.
Lu Y, Cederbaum AI. CYP2E1 and oxidative liver injury by alcohol. Free Radic Biol Med. 2008;44(5):723–38. https://doi.org/10.1016/j.freeradbiomed.2007.11.004.
Gandhi A, Moorthy B, Ghose R. Drug disposition in pathophysiological conditions. Curr Drug Metab. 2012;13(9):1327–44.
Tolman KG, Fonseca V, Dalpiaz A, Tan MH. Spectrum of liver disease in type 2 diabetes and management of patients with diabetes and liver disease. Diabetes Care. 2007;30(3):734–43. https://doi.org/10.2337/dc06-1539.
Wang Z, Hall SD, Maya JF, Li L, Asghar A, Gorski JC. Diabetes mellitus increases the in vivo activity of cytochrome P450 2E1 in humans. Br J Clin Pharmacol. 2003;55(1):77–85.
Kim SK, Novak RF. The role of intracellular signaling in insulin-mediated regulation of drug metabolizing enzyme gene and protein expression. Pharmacol Ther. 2007;113(1):88–120. https://doi.org/10.1016/j.pharmthera.2006.07.004.
Kim YJ, Park HS, Park MH, Suh SH, Pang MG. Oxidative stress-related gene polymorphism and the risk of preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2005;119(1):42–6. https://doi.org/10.1016/j.ejogrb.2004.06.009.
Caro AA, Cederbaum AI. Oxidative stress, toxicology, and pharmacology of CYP2E1. Annu Rev Pharmacol Toxicol. 2004;44:27–42. https://doi.org/10.1146/annurev.pharmtox.44.101802.121704.
Walsh AA, Szklarz GD, Scott EE. Human cytochrome P450 1A1 structure and utility in understanding drug and xenobiotic metabolism. J Biol Chem. 2013;288(18):12932–43. https://doi.org/10.1074/jbc.M113.452953.
Wang T, Chen M, Yan YE, Xiao FQ, Pan XL, Wang H. Growth retardation of fetal rats exposed to nicotine in utero: possible involvement of CYP1A1, CYP2E1, and P-glycoprotein. Environ Toxicol. 2009;24(1):33–42. https://doi.org/10.1002/tox.20391.
Oh SJ, Choi JM, Yun KU, Oh JM, Kwak HC, Oh JG, Lee KS, Kim BH, Heo TH, Kim SK. Hepatic expression of cytochrome P450 in type 2 diabetic Goto-Kakizaki rats. Chemico-Biol Interact. 2012;195(3):173–9. https://doi.org/10.1016/j.cbi.2011.12.010.
Borbas T, Benko B, Dalmadi B, Szabo I, Tihanyi K. Insulin in flavin-containing monooxygenase regulation. Flavin-containing monooxygenase and cytochrome P450 activities in experimental diabetes. Eur J Pharm Sci. 2006;28(1–2):51–8. https://doi.org/10.1016/j.ejps.2005.12.011.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–75.
Reinke LA, Moyer MJ. p-Nitrophenol hydroxylation. A microsomal oxidation which is highly inducible by ethanol. Drug Metab Dispos Biol Fate Chem. 1985;13(5):548–52.
Başaran R, Özdamar ER, Eke BC. A study on the optimization of CYP2E1 enzyme activity in C57Bl/6 mouse brain and liver. FABAD J Pharm Sci. 2012;37(4):191–6.
Burke MD, Thompson S, Elcombe CR, Halpert J, Haaparanta T, Mayer RT. Ethoxy-, pentoxy- and benzyloxyphenoxazones and homologues: a series of substrates to distinguish between different induced cytochromes P-450. Biochem Pharmacol. 1985;34(18):3337–45.
Wills ED. Lipid peroxide formation in microsomes. Relationship of hydroxylation to lipid peroxide formation. Biochem J. 1969;113(2):333–41.
Bishayee S, Balasubramanian AS. Lipid peroxide formation in rat brain. J Neurochem. 1971;18(6):909–20.
Wills ED. Mechanisms of lipid peroxide formation in animal tissues. Biochem J. 1966;99(3):667–76.
Sadi G, Baloglu MC, Pektas MB. Differential gene expression in liver tissues of streptozotocin-induced diabetic rats in response to resveratrol treatment. PLoS One. 2015;10(4):e0124968. https://doi.org/10.1371/journal.pone.0124968.
de Souza Bastos A, Graves DT, de Melo Loureiro AP, Junior CR, Corbi SC, Frizzera F, Scarel-Caminaga RM, Camara NO, Andriankaja OM, Hiyane MI, Orrico SR. Diabetes and increased lipid peroxidation are associated with systemic inflammation even in well-controlled patients. J Diabetes Complicat. 2016;30(8):1593–9. https://doi.org/10.1016/j.jdiacomp.2016.07.011.
He Q, Li JK, Li F, Li RG, Zhan GQ, Li G, Du WX, Tan HB. Mechanism of action of gypenosides on type 2 diabetes and non-alcoholic fatty liver disease in rats. World J Gastroenterol. 2015;21(7):2058–66. https://doi.org/10.3748/wjg.v21.i7.2058.
Morel Y, de Waziers I, Barouki R. A repressive cross-regulation between catalytic and promoter activities of the CYP1A1 and CYP2E1 genes: role of H(2)O(2). Mol Pharmacol. 2000;57(6):1158–64.
Skovso S, Damgaard J, Fels JJ, Olsen GS, Wolf XA, Rolin B, Holst JJ. (2015) Effects of insulin therapy on weight gain and fat distribution in the HF/HS-STZ rat model of type 2 diabetes. Int J Obes. 2005;39(10):1531–8. https://doi.org/10.1038/ijo.2015.92.
Shankar K, Vaidya VS, Apte UM, Manautou JE, Ronis MJ, Bucci TJ, Mehendale HM. Type 1 diabetic mice are protected from acetaminophen hepatotoxicity. Toxicol Sci. 2003;73(2):220–34. https://doi.org/10.1093/toxsci/kfg059.
Kanikarla-Marie P, Jain SK. Hyperketonemia and ketosis increase the risk of complications in type 1 diabetes. Free Radic Biol Med. 2016;95:268–77.
Barnett C, Petrides L, Wilson J, Flatt P, Ioannides C. Induction of rat hepatic mixed-function oxidases by acetone and other physiological ketones: their role in diabetes-induced changes in cytochrome P450 proteins. Xenobiotica. 1992;22(12):1441–50.
Lieber CS. Cyp2e1: from ash to nash. Hepatol Res. 2004;28(1):1–11.
Park SY, Kim CH, Lee JY, Jeon JS, Kim MJ, Chae SH, Kim HC, Oh SJ, Kim SK. Hepatic expression of cytochrome P450 in Zucker diabetic fatty rats. Food Chem Toxicol. 2016;96:244–53. https://doi.org/10.1016/j.fct.2016.08.010.
Woodcroft KJ, Hafner MS, Novak RF. Insulin signaling in the transcriptional and posttranscriptional regulation of CYP2E1 expression. Hepatology. 2002;35(2):263–73. https://doi.org/10.1053/jhep.2002.30691.
Shimojo N, Ishizaki T, Imaoka S, Funae Y, Fujii S, Okuda K. Changes in amounts of cytochrome P450 isozymes and levels of catalytic activities in hepatic and renal microsomes of rats with streptozocin-induced diabetes. Biochem Pharmacol. 1993;46(4):621–7.
Yamazoe Y, Murayama N, Shimada M, Yamauchi K, Kato R. Cytochrome P450 in livers of diabetic rats: regulation by growth hormone and insulin. Arch Biochem Biophys. 1989;268(2):567–75.
Bukan N, Sancak B, Yavuz Ö, Koca C, Tutkun F, Özçelikay AT, Altan N. Lipid peroxidation and scavenging enzyme levels in the liver of streptozotocin-induced diabetic rats. Indian J Biochem Biophys. 2003;40:447–50.
Mukherjee B, Mukherjee JR, Chatterjee M. Lipid peroxidation, glutathione levels and changes in glutathione-related enzyme activities in streptozotocin-induced diabetic rats. Immunol Cell Biol. 1994;72(2):109–14. https://doi.org/10.1038/icb.1994.17.
Niki E. Lipid peroxidation: physiological levels and dual biological effects. Free Radic Biol Med. 2009;47(5):469–84.
Author information
Authors and Affiliations
Contributions
G.K., R.B., and B.C.E. contributed to the concept, design, and analysis of data. E.A.I. has carried out all injections. G.K. and R.B. performed the experimental works. G.K., R.B., and B.C.E. participated in drafting the article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
All procedures used in this study was approved by the Ankara University Local Ethics Committee for Animal Experiments (2010-56-283).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kuzgun, G., Başaran, R., Arıoğlu İnan, E. et al. Effects of insulin treatment on hepatic CYP1A1 and CYP2E1 activities and lipid peroxidation levels in streptozotocin-induced diabetic rats. J Diabetes Metab Disord 19, 1157–1164 (2020). https://doi.org/10.1007/s40200-020-00616-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40200-020-00616-y