Abstract
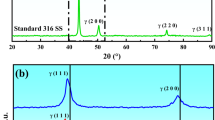
The effect of pH on the electrochemical behaviour and passive film composition of 316L stainless steel in alkaline solutions was studied using electrochemical measurements and a surface analysis method. The critical pH of 12.5 was found for the conversion from pitting corrosion to the oxygen evolution reaction (OER). OER was kinetically faster than pitting corrosion when both reactions could occur, and OER could postpone pitting corrosion. This resulted in pitting being initiated during the reversing scan in the cyclic polarization at the critical pH. According to the X-ray photoelectron spectroscopy analysis, the content of Cr and Mo decreased with pH, while Fe content increased. This induced the degradation of the passive film, which resulted in the higher passive current densities under more alkaline conditions. The selective dissolution of Mo at high pH was found, which demonstrated that the addition of Mo in austenitic stainless steels might not be beneficial to the corrosion resistance of 316L in strong alkaline solutions.


















Similar content being viewed by others
References
Z. Wang, X. Tang, J. Xue, L. Zhang, T. Li, M. Lu, The pitting behavior of stainless steels under SO2 environments with Cl− and F−, in Proceedings of Corrosion 2017, New Orleans, 7–16 March, 2017
A. Singh, K. Stéphenne, Energy Proc. 63, 1678 (2014)
K. Oh, S. Ahn, K. Eom, K. Jung, H. Kwon, Corros. Sci. 79, 34 (2014)
Z. Wang, L. Zhang, X. Tang, Z. Cui, J. Xue, M. Lu, Int. J. Miner. Metall. Mater. 24(8), 943 (2017)
J. Bana, U. Lelek-Borkowska, B. Mazurkiewicz, W. Solarski, Electrochim. Acta 52(18), 5704 (2007)
H. Ge, G. Zhou, W. Wu, Appl. Surf. Sci. 211(1–4), 321 (2003)
D.D. Macdonald, J. Electrochem. Soc. 139(12), 3434 (1992)
Y. Gui, Z.J. Zheng, Y. Gao, Thin Solid Films 599, 64 (2016)
D.G. Li, J.D. Wang, D.R. Chen, Int. J. Hydrog. Energy 39(35), 20105 (2014)
M. Kouril, P. Novak, M. Bojko, Cem. Concr. Res. 40(3), 431 (2010)
S. Fajardo, D.M. Bastidas, M.P. Ryan, M. Criado, D.S. McPhail, J.M. Bastidas, Appl. Surf. Sci. 256(21), 6139 (2010)
S.M. Alvarez, A. Bautista, F. Velasco, Corros. Sci. 53(5), 1748 (2011)
H. Luo, C.F. Dong, X.G. Li, K. Xiao, Electrochim. Acta 64, 211 (2012)
L. Freire, M.J. Carmezim, M.G.S. Ferreira, M.F. Montemor, Electrochim. Acta 55(21SI), 6174 (2010)
L. Freire, M.J. Carmezim, M.G.S. Ferreira, M.F. Montemor, Electrochim. Acta 56(14), 5280 (2011)
Z. Wang, L. Zhang, X. Tang, Z. Zhang, M. Lu, Appl. Surf. Sci. 423, 457 (2017)
Y. Li, Y.F. Cheng, Appl. Surf. Sci. 396, 144 (2017)
W. Fredriksson, S. Malmgren, T. Gustafsson, M. Gorgoi, K. Edstrom, Appl. Surf. Sci. 258(15), 5790 (2012)
B.T. Lu, J.L. Luo, Y.C. Lu, Electrochim. Acta 87, 824 (2013)
S. Refaey, F. Taha, A. El-Malak, Appl. Surf. Sci. 242(1–2), 114 (2005)
A.U. Malik, P.C. Mayan Kutty, N.A. Siddiqi, I.N. Andijani, S. Ahmed, Corros. Sci. 33(11), 1809 (1992)
W. Xu, F. Lyu, Y. Bai, A. Gao, J. Feng, Z. Cai, Y. Yin, Nano Energy 43, 110 (2018)
N. Jiang, B. You, M. Sheng, Y. Sun, Angew. Chem. Int. Edit. 54(21), 6251 (2015)
J. Liu, T. Zhang, G. Meng, Y. Shao, F. Wang, Corros. Sci. 91, 232 (2015)
J. Liu, T. Zhang, H. Li, Y. Zhao, F. Wang, X. Zhang, L. Cheng, K. Wu, J. Electrochem. Soc. 165(7), C328 (2018)
E. Hamada, K. Yamada, M. Nagoshi, N. Makiishi, K. Sato, T. Ishii, K. Fukuda, S. Ishikawa, T. Ujiro, Corros. Sci. 52(12), 3851 (2010)
L. Freire, M.A. Catarino, M.I. Godinho, M.J. Ferreira, M.G.S. Ferreira, A.M.P. Simoes, M.F. Montemor, Cem. Concr. Compos. 34(9), 1075 (2012)
M.F. Montemor, A.M.P. Simoes, M.G.S. Ferreira, M. Da Cunha Belo, Corros. Sci. 41(1), 17 (1999)
J.E. Castle, J.H. Qiu, Corros. Sci. 29(5), 591 (1989)
T. Oshima, Y. Habara, K. Kuroda, ISIJ Int. 47(3), 359 (2007)
H. Luo, C. Dong, K. Xiao, X. Li, RSC Adv. 6(12), 9940 (2016)
Z.H. Jiang, J.P. Han, Y. Li, Mater. Res. Innov. 18(Suppl 5), S5 (2014)
T.J. Mesquita, E. Chauveau, M. Mantel, N. Kinsman, R.P. Nogueira, Mater. Chem. Phys. 126(3), 602 (2011)
I. Betova, M. Bojinov, O. Hyoekyvirta, T. Saario, Corros. Sci. 52(4), 1499 (2010)
C. Zhang, Z. Zhang, L. Liu, Electrochim. Acta 210, 401 (2016)
B. Zhang, S. Hao, J. Wu, X. Li, C. Li, X. Di, Y. Huang, Mater. Charact. 131, 168 (2017)
X. Zhang, J. Zhao, T. Xi, M.B. Shahzad, C. Yang, K. Yang, J. Mater. Sci. Tehcnol. 34, 2149 (2018)
Acknowledgements
This work was supported by the technology projects of State Grid Corporation (No. 52110417000N) and the National Science and Technology Major Project (No. 2016ZX05028-004).
Author information
Authors and Affiliations
Corresponding author
Additional information
Available online at http://link.springer.com/journal/40195
Rights and permissions
About this article
Cite this article
Wang, Z., Zhou, ZQ., Zhang, L. et al. Effect of pH on the Electrochemical Behaviour and Passive Film Composition of 316L Stainless Steel. Acta Metall. Sin. (Engl. Lett.) 32, 585–598 (2019). https://doi.org/10.1007/s40195-018-0794-5
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40195-018-0794-5