Abstract
Purpose of the Review
Peripheral nerve injuries are common, debilitating, and costly. The human body’s innate regenerative capacity is slow, and nerves are often misguided. The purpose of this article is to review a specific cellular, regenerative engineering technique that holds promise for the treatment of peripheral nerve injuries.
Recent Findings
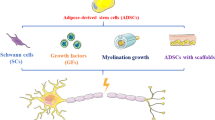
Over the past several decades, research has focused on the utilization of stem cells for peripheral nerve repair. More recently, stem cells collected from adipose tissue (adipose-derived stem cells or ADSCs) have gained traction due to their relative ease of collection and differentiation potential. Both undifferentiated and Schwann cell-like differentiated ADSCs have been used to seed conduits with variable results.
Summary
Technical and ethical issues surrounding stem cells’ self-expansion potential and genetic makeup exist. Ultimately, randomized control trials and FDA approval will be required before widespread clinical translation in the US is realized.

(Reproduced with permission from Hindawi Publishing Corporation)

(Reproduced with permission from Elsevier)

(Reproduced with permission from The Royal Society)

(Reproduced with permission from Springer)

(Reproduced with permission from Springer)
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Taylor CA, et al. The incidence of peripheral nerve injury in extremity trauma. Am J Phys Med Rehabil. 2008;87(5):381–5.
Noble J, et al. Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries. J Trauma. 1998;45(1):116–22.
Dalamagkas K, Tsintou M, Seifalian A. Advances in peripheral nervous system regenerative therapeutic strategies: a biomaterials approach. Mater Sci Eng C. 2015;65:425–32.
DiMaggio C, et al. Traumatic injury in the United States: in-patient epidemiology 2000–2011. Injury. 2016;47(7):1393–403.
Grinsell D, Keating CP. Peripheral nerve reconstruction after injury: a review of clinical and experimental therapies. BioMed Res Int. 2014;2014:13.
Conforti L, Gilley J, Coleman MP. Wallerian degeneration: an emerging axon death pathway linking injury and disease. Nat Publ Group. 2014;15(6):394–409.
Mirsky R, Lloyd AC. Schwann cells: development and role in nerve repair. Cold Spring Harbor Perspect Biol. 2016;7:1–16.
• Cattin, A-L, et al. Macrophage-induced blood vessels guide Schwann cell-mediated regeneration of peripheral nerves. Cell. 2015;162(5):1127–39.
Cattin AL, Lloyd AC. The multicellular complexity of peripheral nerve regeneration. Curr Opin Neurobiol. 2016;39:38–46.
Seddon HJ. A Classification of Nerve Injuries. Br Med J. 1942;2(4260):237–9.
Sunderland S. A classification of peripheral nerve injuries producing loss of function. Brain. 1951;74(4):491–516.
Houschyar KS, et al. The role of current techniques and concepts in peripheral nerve repair. Plast Surg Int. 2016;2016:1–8.
Lundborg G. A 25-year perspective of peripheral nerve surgery: evolving neuroscientific concepts and clinical significance. J Hand Surg. 2000;25(3):391–414.
Siemionow M, Bozkurt M, Zor F. Regeneration and repair of peripheral nerves with different biomaterials: review. Microsurgery. 2010;30(7):574–88.
Muheremu A, Ao Q. Past, present, and future of nerve conduits in the treatment of peripheral nerve injury. BioMed Res Int. 2015;. doi:10.1155/2015/237507.
Safa B, Buncke G. Autograft substitutes. conduits and processed nerve allografts. Hand Clin. 2016;32(2):127–40.
Kehoe S, Zhang XF, Boyd D. FDA approved guidance conduits and wraps for peripheral nerve injury: a review of materials and efficacy. Injury. 2012;43(5):553–72.
Pabari A, et al. Modern surgical management of peripheral nerve gap. J Plast Reconstr Aesthet Surg. 2010;63(12):1941–8.
Wolfe SW, et al. Use of bioabsorbable nerve conduits as an adjunct to brachial plexus neurorrhaphy. J Hand Surg. 2012;37(10):1980–5.
Deumens R, et al. Repairing injured peripheral nerves: bridging the gap. Prog Neurobiol. 2010;92(3):245–76.
Deal ND, Griffin JW, Hogan MV. Nerve conduits for nerve repair or reconstruction. J Am Acad Orthop Surg. 2012;20(2):63–8.
Daly W, et al. A biomaterials approach to peripheral nerve regeneration: bridging the peripheral nerve gap and enhancing functional recovery. J R Soc Interface. 2012;9(67):202–21.
Hoben G, et al. Comparison of acellular nerve allograft modification with Schwann cells or VEGF. Hand. 2015;10(3):396–402.
• Zhou, L-N., et al. Co-graft of bone marrow stromal cells and Schwann cells into acellular nerve scaffold for sciatic nerve regeneration in rats. J Oral Maxillofa Surg. 2015;73(8):1651–1660.
Pereira Lopes FR, et al. Bone marrow stromal cells and resorbable collagen guidance tubes enhance sciatic nerve regeneration in mice. Exp Neurol. 2006;198(2):457–68.
Dai R, et al. Adipose-derived stem cells for tissue engineering and regenerative medicine applications. Stem Cells Int. 2016;2016:6737345.
Raff M. Adult Stem Cell Plasticity: fact or Artifact? Annu Rev Cell Dev Biol. 2003;19(1):1–22.
Kern S, et al. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24(5):1294–301.
Chen C-J, et al. Transplantation of bone marrow stromal cells for peripheral nerve repair. Exp Neurol. 2007;204(1):443–53.
Keilhoff G, et al. Transdifferentiated mesenchymal stem cells as alternative therapy in supporting nerve regeneration and myelination. Cell Mol Neurobiol. 2006;26(7):1233–50.
Wang D, et al. Bridging small-gap peripheral nerve defects using acellular nerve allograft implanted with autologous bone marrow stromal cells in primates. Brain Res. 2008;1188:44–53.
Dezawa M, et al. Sciatic nerve regeneration in rats induced by transplantation of in vitro differentiated bone-marrow stromal cells. Eur J Neurosci. 2001;14(11):1771–6.
Tohill M, et al. Rat bone marrow mesenchymal stem cells express glial markers and stimulate nerve regeneration. Neurosci Lett. 2004;362(3):200–3.
Walsh S, Midha R. Practical considerations concerning the use of stem cells for peripheral nerve repair. Neurosurg Focus. 2009;26(2):E2.
Zuk PA, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–28.
Kim EH, Heo CY. Current applications of adipose-derived stem cells and their future perspectives. World J Stem Cells. 2014;6(1):65–8.
Strem BM, et al. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med. 2005;54(3):132–41.
Locke M, Windsor J, Dunbar PR. Human adipose-derived stem cells: isolation, characterization and applications in surgery. ANZ J Surg. 2009;79(4):235–44.
Kobolak J, et al. Mesenchymal stem cells: Identification, phenotypic characterization, biological properties and potential for regenerative medicine through biomaterial micro-engineering of their niche. Methods. 2015;99:62–8.
• Mildmay-White A, Khan W. Cell surface markers on adipose-derived stem cells: a systematic review. Curr Stem Cell Res Ther. 2016;11:1.
Jang S, et al. Functional neural differentiation of human adipose tissue-derived stem cells using bFGF and forskolin. BMC Cell Biol. 2010;11(1):1–13.
Jafarzadeh N, et al. Oxytocin improves neuronal differentiation of adipose tissue-derived stem cells. Cell J. 2013;15:48.
Zack-Williams SDL, Butler PE, Kalaskar DM. Current progress in use of adipose derived stem cells in peripheral nerve regeneration. World J Stem Cells. 2015;7(1):51–64.
Ramasamy SK, Lenka N. Notch exhibits ligand bias and maneuvers stage-specific steering of neural differentiation in embryonic stem cells. Mol Cell Biol. 2010;30(8):1946–57.
•• Widgerow AD. et al. Neuromodulatory nerve regeneration: adipose tissue-derived stem cells and neurotrophic mediation in peripheral nerve regeneration. J Neurosci Res. 2013;91(12):1517–1524.
Faroni A, Terenghi G, Reid AJ. Chapter five—adipose-derived stem cells and nerve regeneration: promises and pitfalls. In: Geuna S, Bruno B, editors. International Review of Neurobiology, I.P.P.T. London: Academic Press; 2013. p. 121–136.
Kingham PJ, et al. Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp Neurol. 2007;207(2):267–74.
di Summa PG, et al. Adipose-derived stem cells enhance peripheral nerve regeneration. J Plast Reconstr Aesthet Surg. 2010;63(9):1544–52.
di Summa PG, et al. Long-term in vivo regeneration of peripheral nerves through bioengineered nerve grafts. Neuroscience. 2011;181:278–91.
Wang Y, et al. Recellularized nerve allografts with differentiated mesenchymal stem cells promote peripheral nerve regeneration. Neurosci Lett. 2012;514(1):96–101.
Liu G, et al. Transplantation of adipose-derived stem cells for peripheral nerve repair. Int J Mol Med. 2011;28(4):565–72.
Santiago LY, et al. Delivery of adipose-derived precursor cells for peripheral nerve repair. Cell Transplant. 2009;18(2):145–58.
Shen C-C, Yang Y-C, Liu B-S. Peripheral nerve repair of transplanted undifferentiated adipose tissue-derived stem cells in a biodegradable reinforced nerve conduit. J Biomed Mater Res. 2012;100A(1):48–63.
Xu Y, et al. A silk fibroin/collagen nerve scaffold seeded with a co-culture of Schwann Cells and adipose-derived stem cells for Sciatic nerve regeneration. PLoS ONE. 2016;11(1):e0147184.
Reichenberger MA, et al. ADSCs in a fibrin matrix enhance nerve regeneration after epineural suturing in a rat model. Microsurgery. 2016;36(6):491–500.
• Matsumine H, et al. Facial-nerve regeneration ability of a hybrid artificial nerve conduit containing uncultured adipose-derived stromal vascular fraction: an experimental study. Microsurgery. 2016.
Mohammadi R, et al. Repair of nerve defect with chitosan graft supplemented by uncultured characterized stromal vascular fraction in streptozotocin induced diabetic rats. Int J Surg. 2014;12(1):33–40.
Mohammadi R, et al. Transplantation of uncultured omental adipose-derived stromal vascular fraction improves sciatic nerve regeneration and functional recovery through inside-out vein graft in rats. J Trauma Acute Care Surg. 2012;72(2):390–6.
Cosmetic surgery national data bank statistics for 2013. September 20, 2016; http://www.surgery.org/sites/default/files/Stats2013_3.pdf.
Brattain K. Analysis of the peripheral nerve repair market in the United States. 2013.
Wilson D. A troubled past? Reassessing ethics in the history of tissue culture. Health Care Anal. 2016;24:246–59.
Harbaugh JT. Do you own your 3D bioprinted body?: analyzing property issues at the intersection of digital information and biology. Am J Law Med. 2014;41(1):167–89.
ASTM E23-12c Standard test methods for notched bar impact testing of metallic materials. 2012;http://dx.doi.org/10.1520/E0023-12C.
Guidance for industry. regulation of human cells, tissues, and cellular and tissue-based products (HCT/Ps). 2007; http://www.fda.gov/downloads/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Tissue/ucm062592.pdf.
Vériter S, et al. Human adipose-derived mesenchymal stem cells in cell therapy: safety and feasibility in different “hospital exemption” clinical applications. PLoS ONE. 2015;10(10):e0139566.
Bowles AC, Scruggs BA, Bunnell B. Mesenchymal stem cell-based therapy in a mouse model of experimental autoimmune encephalomyelitis (EAE). In: Animal models for stem cell therapy. Methods in molecular biology, vol. 1213. New York: Springer; 2014. p. 303–19.
Acknowledgements
Dr. Ravnic is supported by a Penn State Department of Surgery Grant and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under BIRCWH award number K12HD055882, “Career Development Program in Women’s Health Research at Penn State.” The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Traumatic Brain Injury Surgery.
Rights and permissions
About this article
Cite this article
Leberfinger, A.N., Ravnic, D.J., Payne, R. et al. Adipose-Derived Stem Cells in Peripheral Nerve Regeneration. Curr Surg Rep 5, 5 (2017). https://doi.org/10.1007/s40137-017-0169-2
Published:
DOI: https://doi.org/10.1007/s40137-017-0169-2