Abstract
Purpose of Review
This review summarizes recent advances in biomarker discovery and treatment of renal and extrarenal IgA vasculitis (IgAV), formerly known as Henoch-Schönlein purpura.
Recent Findings
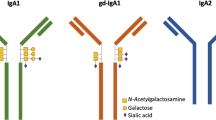
Elevated levels of urinary IgA and serum galactose-deficient IgA1 at presentation are associated with risk of developing renal involvement. Elevated neutrophil to lymphocyte ratios at presentation may also indicate increased risk of renal but also gastrointestinal involvement. Rituximab has been beneficial in retrospective studies in the treatment of corticosteroid refractory IgAV nephritis. Although large studies have not conclusively demonstrated the superiority of any one steroid-sparing agent, MMF has emerged as a common steroid-sparing agent to help with disease control, while many other patients initially treated with steroids have also been switched to calcineurin inhibitors to control the glomerular disease.
Summary
Candidate biomarkers at presentation, specifically urinary IgA and neutrophil to lymphocyte ratios, may be helpful in risk stratifying patients at risk of developing IgAV nephritis. Further research is required in order to determine any recommendations for change in monitoring or management based on information gained from biomarkers. Guidelines recommend treatment of IgAV nephritis with corticosteroids, oral or intravenous depending on severity, renin–angiotensin–aldosterone system blockade, and the addition of cyclophosphamide for severe nephritis. For second-line therapies, in addition to azathioprine and mycophenolate mofetil, there is growing evidence to consider rituximab and calcineurin inhibitors.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Piram M, Maldini C, Biscardi S, et al. Incidence of IgA vasculitis in children estimated by four-source capture-recapture analysis: a population-based study. Rheumatology (Oxford). 2017;56:1358–66.
Ozen S, Pistorio A, Iusan SM, et al. EULAR, PRINTO, PRES criteria for Henoch-Schönlein purpura, childhood polyarteritis nodosa, childhood Wegener granulomatosis and childhood Takayasu arteritis: Ankara 2008 Part II: Final classification criteria. Ann Rheum Dis. 2010;69:798–806.
Shim JO, Han K, Park S, et al. Ten-year nationwide population-based survey on the characteristics of children with Henoch-Schӧnlein purpura in Korea. J Korean Med Sci. 2018;33:e174.
Narchi H. Risk of long term renal impairment and duration of follow up recommended for Henoch-Schonlein purpura with normal or minimal urinary findings: a systematic review. Arch Dis Child. 2005;90:916–20.
Davin JC, Coppo R. Henoch-Schönlein purpura nephritis in children. Nat Rev Nephrol. 2014;10:563–73.
Ronkainen J, Nuutinen M, Koskimies O. The adult kidney 24 years after childhood Henoch-Schönlein purpura: a retrospective cohort study. Lancet. 2002;360:666–70.
Heineke MH, Ballering AV, Jamin A, et al. New insights in the pathogenesis of immunoglobulin A vasculitis (Henoch-Schönlein purpura). Autoimmun Rev. 2017;16:1246–53.
Hetland CE, Susrud KS, Lindahl KH, et al. Henoch-Schönlein purpura: a literature review. Acta Derm Venereol. 2017;97:1160–6.
Jauhola O, Ronkainen J, Koskimies O, et al. Clinical course of extrarenal symptoms in Henoch-Schonlein purpura: a 6-month prospective study. Arch Dis Child. 2010;95:871–6.
Rajagopala S, Shobha V, Devaraj U, et al. Pulmonary hemorrhage in Henoch-Schönlein purpura: case report and systematic review of the English literature. Semin Arthritis Rheum. 2013;42:391–400.
• Suzuki H, Yasutake J, Makita Y, et al. IgA nephropathy and IgA vasculitis with nephritis have a shared feature involving galactose-deficient IgA1-oriented pathogenesis. Kidney Int. 2018;93:700-705.. (Kidney biopsies from 97 patients assessed via immunohistology staining for presence of galactose-deficient IgA1. Only IgA nephropathy and IgA vasculitis patients demonstrated staining of galactose-deficient IgA1. Samples from patients with lupus nephritis or membranous nephropathy did not have any staining of galactose-deficient IgA1, suggesting shared pathologic link in IgA nephropathy and vasculitis.)
Pillebout E, Jamin A, Ayari H, et al. Biomarkers of IgA vasculitis nephritis in children. PLoS One. 2017;12:e0188718.
Berthelot L, Jamin A, Viglietti D, et al. Value of biomarkers for predicting immunoglobulin A vasculitis nephritis outcome in an adult prospective cohort. Nephrol Dial Transplant. 2018;33:1579–90.
Michailidou D, Mustelin T, Lood C. Role of neutrophils in systemic vasculitides Front Immunol. 2020;11:619705.
Özdemir ZC, Çetin N, Kar YD, et al. Hemotologic indices for predicting internal organ involvement in Henoch-Schönlein purpura (IgA vasculitis). J Pediatr Hematol Oncol. 2020;42:e46–9.
Jaszczura M, Góra A, Grzywna-Rozenek E, et al. Analysis of neutrophil to lymphocyte ratio, platelet to lymphocyte ratio and mean platelet volume to platelet count ratio in children with acute stage of immunoglobulin A vasculitis and assessment of their suitability for predicting the course of the disease. Rheumatol Int. 2019;39:869–78.
Nagy GR, Kemény L, Bata-Csörgő Z. Neutrophil-to-lymphocyte ratio: a biomarker for predicting systemic involvement in adult IgA vasculitis patients. J Eur Acad Dermatol Venereol. 2017;31:1033–7.
George RM, Inamadar AC, Janagond AB. Neutrophil-to-lymphocyte ratio: a biomarker for predicting systemic involvement in IgA vasculitis. Indian Journal of Rheumatology. 2020;15:187.
Hočevar A, Tomšič M, Jurčić V, et al. Predicting gastrointestinal and renal involvement in adult IgA vasculitis. Arthritis Res Ther. 2019;21:1–7.
• Fu W, Ye W, Liu X, et al. Meta-analysis of the neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios in Henoch-Schonlein purpura and its complications. Int Immunopharmacol. 2021;94:107454.. (Meta-analysis of 1691 patients with IgA vasculitis and 563 health controls found significantly higher neutrophil-to-lymphocyte ratio (NLR) in patients with IgA vasculitis. This study also noted that a higher NLR was predictive of both renal and/or gastrointestinal complications in patients with IgA vasculitis. Data from this meta-analysis support the role for future research into the use and potential clinical benefits of NLR as a readily available biomarker in IgA vasculitis.•)
Nakagawa M, Terashima T, D’yachkova Y, et al. Glucocorticoid-induced granulocytosis: contribution of marrow release and demargination of intravascular granulocytes. Circulation. 1998;98:2307–13.
Toraman A, Neşe N, Özyurt BC, et al. Association between neutrophil-lymphocyte & platelet lymphocyte ratios with prognosis & mortality in rapidly progressive glomerulonephritis. Indian J Med Res. 2019;150:399.
Ahn SS, Jung SM, Song JJ, et al. Neutrophil to lymphocyte ratio at diagnosis can estimate vasculitis activity and poor prognosis in patients with ANCA-associated vasculitis: a retrospective study. BMC Nephrol. 2018;19:1–7.
Huang L, Shen C, Zhong Y, et al. The association of neutrophil-to-lymphocyte ratio with all-cause mortality in Chinese patients with MPO-ANCA associated vasculitis. Clin Exp Med. 2020;20:401–8.
Li Q, Chen P, Shi S, et al. Neutrophil to lymphocyte ratio as an independent inflammatory indicator of poor prognosis in IgA nephropathy. Int Immunopharmacol. 2020;87:106811.
Yang H, Zhang W, Li Y, et al. Neutrophil-to-lymphocyte ratio: an effective predictor of corticosteroid response in IgA nephropathy. Int Immunopharmacol. 2019;74:105678.
López-Mejías R, Carmona FD, Castañeda S, et al. A genome-wide association study suggests the HLA class II region as the major susceptibility locus for IgA vasculitis. Sci Rep. 2017;7:1–6.
Weiss PF, Feinstein JA, Luan X, et al. Effects of corticosteroid on Henoch-Schönlein purpura: a systematic review. Pediatrics. 2007;120:1079–87.
Uluca Ü, Ece A, Şen V, et al. Management of intestinal bleeding with single-dose cyclophosphamide in Henoch-Schönlein purpura. Pediatr Int. 2015;57:498–500.
Wang H, Zhang B, Li S, et al. Clinical outcome in pediatric refractory gastrointestinal Henoch-Schönlein purpura treated with mycophenolate mofetil. Eur J Pediatr. 2020;179:1361–6.
Cherqaoui B, Chausset A, Stephan JL, et al. Intravenous immunoglobulins for severe gastrointestinal involvement in pediatric Henoch-Schönlein purpura: a French retrospective study. Arch Pediatr. 2016;23:584–90.
Ronkainen J, Koskimies O, Ala-Houhala M, et al. Early prednisone therapy in Henoch-Schönlein purpura: a randomized, double-blind, placebo-controlled trial. J Pediatr. 2006;149:241–7.
Lee KH, Park JH, Kim DH, et al. Dapsone as a potential treatment option for Henoch-Schönlein purpura (HSP). Med Hypotheses. 2017;108:42–5.
Nothhaft M, Klepper J, Kneitz H, et al. Hemorrhagic bullous Henoch-Schönlein purpura: case report and review of the literature. Front Pediatr. 2019;6:413.
Ma Y, Zhang S, Chen J, et al. Henoch-Schönlein purpura with scrotal involvement: a case report and literature review. J Pediatr Hematol Oncol 2021.
Fikse DJ, Grenz PM, Wheatley SM, et al. Posterior reversible encephalopathy syndrome associated with Henoch Schonlein purpura in a pediatric patient. Am J Emerg Med. 2021;43:291-e.5.
Wen YK, Yang Y, Chang CC. Cerebral vasculitis and intracerebral hemorrhage in Henoch-Schönlein purpura treated with plasmapheresis. Pediatr Nephrol. 2005;20:223–5.
Cattran DC, Feehally J, Cook HT, et al. Kidney disease: improving global outcomes KDIGO glomerulonephritis work group KDIGO clinical practice guideline for glomerulonephritis. Kidney International Supplements. 2012;2(139):274.
Hahn D, Hodson EM, Willis NS, et al. Interventions for preventing and treating kidney disease in Henoch-Schönlein purpura (HSP). Cochrane Database Syst Rev 2015, (8):CD005128. doi:CD005128.
• Selewski DT, Ambruzs JM, Appel GB, et al. Clinical characteristics and treatment patterns of children and adults with IgA nephropathy or IgA vasculitis: findings from the CureGN study. Kidney Int Rep. 2018;3:1373–84.. (Multicenter cohort study of adult and pediatric patients with IgA nephropathy and IgA vasculitis. Patients with IgA vasculitis were more likely than those with IgA nephropathy to receive corticosteroids. 75% of patients with IgA vasculitis with nephritis received treatment with corticosteroids in this cohort, and this data provides insight into the common practice for this disease in the modern day.)
• Ozen S, Marks SD, Brogan P, et al. European consensus-based recommendations for diagnosis and treatment of immunoglobulin A vasculitis-the SHARE initiative. Rheumatology (Oxford). 2019;58:1607–16.. (Single Hub and Access point for paediatric Rheumatology in Europe (SHARE) collaborative provided 7 diagnostic and 19 treatment recommendations for children with IgA vasculitis based on expert agreement. Oral corticosteroids 1–2 mg/kg are recommended for mild and moderate kidney disease, and IV methylprednisolone is recommended for severe kidney involvement. These guidelines provide an updated framework for corticosteroid use in IgA vasculitis with glomerular involvement.)
Flynn JT, Smoyer WE, Bunchman TE, et al. Treatment of Henoch-Schönlein purpura glomerulonephritis in children with high-dose corticosteroids plus oral cyclophosphamide. Am J Nephrol. 2001;21:128–33.
Kawasaki Y, Suzuki J, Suzuki H. Efficacy of methylprednisolone and urokinase pulse therapy combined with or without cyclophosphamide in severe Henoch-Schoenlein nephritis: a clinical and histopathological study. Nephrol Dial Transplant. 2004;19:858–64.
Tarshish P, Bernstein J, Edelmann CM Jr. Henoch-Schönlein purpura nephritis: course of disease and efficacy of cyclophosphamide. Pediatr Nephrol. 2004;19:51–6.
Pillebout E, Alberti C, Guillevin L, et al. Addition of cyclophosphamide to steroids provides no benefit compared with steroids alone in treating adult patients with severe Henoch Schönlein Purpura. Kidney Int. 2010;78:495–502.
Shah S, Hata J. A rare and severe presentation of Henoch-Schönlein purpura in an adolescent with crescentic glomerulonephritis, arrhythmia, acute gastrointestinal bleed, and neurological complications. Cureus 2021, 13.
Koskela M, Jahnukainen T, Endén K, et al. Methylprednisolone or cyclosporine a in the treatment of Henoch-Schönlein nephritis: a nationwide study. Pediatr Nephrol. 2019;34:1447–56.
Jauhola O, Ronkainen J, Autio-Harmainen H, et al. Cyclosporine A vs methylprednisolone for Henoch-Schönlein nephritis: a randomized trial. Pediatr Nephrol. 2011;26(2159):2166.
Zhang DF, Hao GX, Li CZ, et al. Off-label use of tacrolimus in children with Henoch-Schönlein purpura nephritis: a pilot study. Arch Dis Child. 2018;103:772–5.
Hackl A, Becker JU, Körner LM, et al. Mycophenolate mofetil following glucocorticoid treatment in Henoch-Schönlein purpura nephritis: the role of early initiation and therapeutic drug monitoring. Pediatr Nephrol. 2018;33:619–29.
Du Y, Hou L, Zhao C, et al. Treatment of children with Henoch-Schönlein purpura nephritis with mycophenolate mofetil. Pediatr Nephrol. 2012;27:765–71.
Lu Z, Song J, Mao J, et al. Evaluation of mycophenolate mofetil and low-dose steroid combined therapy in moderately severe Henoch-Schönlein purpura nephritis. Med Sci Monit. 2017;23:2333–9.
Meadow SR, Glasgow EF, White R, et al. Schönlein Henoch nephritis QJM An. Int J Meds. 1972;41(241):260.
Ren P, Han F, Chen L, et al. The combination of mycophenolate mofetil with corticosteroids induces remission of Henoch-Schönlein purpura nephritis. Am J Nephrol. 2012;36:271–7.
Han F, Chen LL, Ren PP, et al. Mycophenolate mofetil plus prednisone for inducing remission of Henoch-Schönlein purpura nephritis: a retrospective study. J Zhejiang Univ Sci B. 2015;16:772–9.
Schinzel V, Fernandez JD, Clemente G, et al. The profile and clinical outcomes of patients with renal involvement due to IgA vasculitis: is azathioprine a good option for treatment? Adv Rheumatol. 2019;59:21–x.
Shin JI, Park JM, Shin YH, et al. Can azathioprine and steroids alter the progression of severe Henoch-Schönlein nephritis in children? Pediatr Nephrol. 2005;20:1087–92.
Crayne CB, Eloseily E, Mannion ML, et al. Rituximab treatment for chronic steroid-dependent Henoch-Schonlein purpura: 8 cases and a review of the literature. Pediatr Rheumatol Online J. 2018;16:71–2.
Lundberg S, Westergren E, Smolander J, et al. B cell-depleting therapy with rituximab or ofatumumab in immunoglobulin A nephropathy or vasculitis with nephritis. Clin Kidney J. 2017;10:20–6.
Fenoglio R, Sciascia S, Naretto C, et al. Rituximab in severe immunoglobulin-A vasculitis (Henoch-Schönlein) with aggressive nephritis. Clin Exp Rheumatol. 2020;38(Suppl 124):195–200.
Maritati F, Fenoglio R, Pillebout E, et al. Brief report: rituximab for the treatment of adult-onset IgA vasculitis (Henoch-Schönlein). Arthritis Rheumatol. 2018;70:109–14.
Hernández-Rodríguez J, Carbonell C, Mirón Canelo JA, et al. Rituximab treatment for IgA vasculitis: a systematic review. Autoimmun Rev. 2020;19:102490.
Inoue CN, Chiba Y, Morimoto T, et al. Tonsillectomy in the treatment of pediatric Henoch-Schönlein nephritis. Clin Nephrol. 2007;67:298–305.
Kanai H, Sawanobori E, Kobayashi A, et al. Early treatment with methylprednisolone pulse therapy combined with tonsillectomy for heavy proteinuric Henoch-Schönlein purpura nephritis in children. Nephron Extra. 2011;1:101–11.
Inoue CN, Matsutani S, Ishidoya M, et al. Impact of periodontal treatment in combination with tonsillectomy plus methylprednisolone pulse therapy and angiotensin blockade for pediatric IgA nephropathy. Clin Nephrol. 2012;77:137–45.
Umeda C, Fujinaga S, Endo A, et al. Preventive effect of tonsillectomy on recurrence of Henoch-Schönlein purpura nephritis after intravenous methylprednisolone pulse therapy. Tohoku J Exp Med. 2020;250:61–9.
Suzuki H, Yasutake J, Makita Y, et al. IgA nephropathy and IgA vasculitis with nephritis have a shared feature involving galactose-deficient IgA1-oriented pathogenesis. Kidney Int. 2018;93:700–5.
• Liu LJ, Yang YZ, Shi SF, et al. Effects of hydroxychloroquine on proteinuria in IgA nephropathy: a randomized controlled trial. Am J Kidney Dis. 2019;74:15–22.. (Double-blind, randomized, placebo-controlled trial of hydroxychloroquine (HCQ) in addition to angiotensin converting enzyme (ACE) inhibitor for control of proteinuria in IgA nephropathy. At 6 months, patients with HCQ and ACE inhibitors had significantly improved proteinuria compared to the ACE inhibitor and placebo group. This study highlights the antiproteinuric potential of HCQ in IgA nephropathy and given evidence of some shared pathogenesis with IgA vasculitis, raises interest in studying this medication in this disease.)
Yang YZ, Chen P, Liu LJ, et al. Comparison of the effects of hydroxychloroquine and corticosteroid treatment on proteinuria in IgA nephropathy: a case-control study. BMC Nephrol. 2019;20:297–6.
Casian A, Sangle SR, D’Cruz DP. New use for an old treatment: hydroxychloroquine as a potential treatment for systemic vasculitis. Autoimmun Rev. 2018;17:660–4.
Bai L, Li H, Li J, et al. Immunosuppressive effect of artemisinin and hydroxychloroquine combination therapy on IgA nephropathy via regulating the differentiation of CD4+ T cell subsets in rats. Int Immunopharmacol. 2019;70:313–23.
Bai L, Li J, Li H, et al. Renoprotective effects of artemisinin and hydroxychloroquine combination therapy on IgA nephropathy via suppressing NF κB signaling and NLRP3 inflammasome activation by exosomes in rats. Biochem Pharmacol. 2019;169:113619.
Wu SH, Liao PY, Chen XQ, et al. Add-on therapy with montelukast in the treatment of Henoch-Schönlein purpura. Pediatr Int. 2014;56:315–22.
Patel DM, Cantley L, Moeckel G, et al. IgA vasculitis complicated by acute kidney failure with thrombotic microangiopathy: successful use of eculizumab. J Nephrol 2021.
Selvaskandan H, Kay Cheung C, Dormer J, et al. Inhibition of the lectin pathway of the complement system as a novel approach in the management of IgA vasculitis-associated nephritis. Nephron. 2020;144:453–8.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Park, J., Berard, R.A., Grimmer, J. et al. IgA Vasculitis: a Review and Update on the Management of Renal and Extrarenal Disease, Highlighting What’s New for Biomarkers and Treatment. Curr Pediatr Rep 9, 118–126 (2021). https://doi.org/10.1007/s40124-021-00247-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40124-021-00247-8