Abstract
Introduction
The aim of our analysis was to compare the effectiveness of the XEN45 gel stent implantation in patients without and with prior glaucoma intervention.
Methods
Retrospective analysis including 148 medical records of consecutive glaucoma eyes without prior glaucoma intervention (group A, n = 45) or with prior glaucoma intervention (group B, n = 103). Follow-up data up to 12 months after XEN45 gel implantation were available for all eyes.
Results
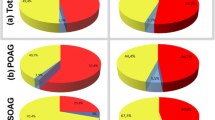
At 12 months, qualified success (IOP reduction of ≥ 20% and IOP < 18 mmHg without and with medication) was achieved in 76% of eyes in group A and in 72% of eyes in group B; corresponding values for complete success (IOP reduction of ≥ 20% and IOP < 18 mmHg without medication) were 56% and 55%. Mean IOP was significantly reduced by 58% from 36.0 ± 10.7 mmHg preoperatively to 14.2 ± 3.4 mmHg at 12 months in group A (p = 0.000) and by 53% from 31.6 ± 8.9 mmHg to 14.3 ± 4.2 mmHg and in group B (p = 0.000). The mean number of hypotensive medications had significantly decreased from 3.6 ± 0.8 at baseline to 0.3 ± 0.7 medications in group A (p = 0.000) and from 3.0 ± 1.0 to 0.3 ± 0.7 medications in group B (p = 0.000). Needling was required in 29% of eyes in group A and in 35% of group B within 12 months. No statistically significant differences were observed between eyes without and with prior glaucoma intervention.
Conclusion
The 1-year results of our retrospective analysis indicate that patients without and with previous glaucoma intervention can benefit from XEN45 gel stent implantation. Both groups achieved significant and similar reductions in IOP and hypotensive medication, with a slight trend towards greater reductions in eyes without prior glaucoma intervention. Further controlled prospective studies with longer follow-ups are required.
Funding
Editorial support and article processing charges were funded by Allergan.
Similar content being viewed by others
Introduction
Glaucoma is one of the most common causes of blindness and currently affects more than 64 million people worldwide [1]. This chronic, progressive eye disease damages the nerve fiber layer and the optical nerve and finally leads to loss of visual field and visual acuity [2]. Since an increased intraocular pressure (IOP) is considered a significant risk factor, treatment requires an effective, long-term reduction in IOP for which various treatment methods are available today [3]. Surgical treatments are necessary if topical ocular hypotensive therapy is not sufficient, not adequately applied, or not tolerated [2]. While trabeculectomy is still the gold standard filtering surgery and shows good efficacy, it requires a strict postoperative follow-up and brings with it a range of intra- and postoperative complications [4]. In order to make less invasive filtrating therapy possible, various minimally invasive glaucoma surgery (MIGS) devices have been developed in recent years, all of which can be implanted ab interno, but which differ regarding the addressed drainage path of the aqueous humor [5]. The XEN45 gel implant (XEN45) (Allergan, Dublin, Ireland) is the only commercially available MIGS device that creates a permanent shunt from the anterior chamber to the subconjunctival space, thus bypassing the natural drainage pathways which often are obstructed in glaucoma patients [6,7,8]. The XEN45 gel implant is made of hydrophilic gelatin glutaraldehyde, making it non-degrading, with good biocompatibility and tolerability [7, 9]. The dimensions of the tube (6 mm in length; 45 µm inner diameter) were designed to maximize long-term aqueous humor drainage while minimizing the risk of hypotension [7, 9,10,11]. At present, it is approved in Europe and USA with slightly different indications. In Europe, the XEN45 gel stent is indicated in patients with primary open-angle glaucoma (POAG) who have failed previous medical treatments, while the US indication includes management of refractory glaucomas, including cases where previous surgical treatment has failed, cases of POAG, and pseudoexfoliative or pigmentary glaucomas with open angle that are unresponsive to maximum tolerated medical therapy [12, 13]. Clinical studies have shown that the XEN45 can be implanted with and without combination of phacoemulsification and effectively reduces IOP while maintaining a good safety profile [14,15,16,17,18,19]. Moreover, an international multicenter retrospective study compared XEN gel stent implantation and trabeculectomy in 354 eyes with refractory glaucoma and no prior incisional filtering surgery. The results demonstrated that there was no difference in efficacy, risk of failure, and safety profile between the two surgical procedures [20].
Most of the studies included patients who received the XEN45 stent as an initial surgical therapy. However, since glaucoma is a lifelong chronic disease, there are many patients with failed previous incisional glaucoma therapies who might benefit from an XEN45 stent implantation. With regard to trabeculectomy, however, a previous study showed that eyes with initial trabeculectomy (n = 75) had a statistically significantly higher success rate and required statistically fewer medications at 3 years postoperatively than those that underwent repeat trabeculectomy (n = 75) [21]. Even though individual studies on the XEN45 gel stent included patients with previous glaucoma interventions [14, 15, 22], to the best of our knowledge no comparative analysis has yet been published in a peer review article.
The aim of our retrospective analysis was to compare IOP-lowering and medication-reducing effects of the XEN45 gel stent in glaucoma patients without and with prior glaucoma interventions in order to investigate whether both patient groups benefit from XEN45 gel implantation.
Methods
We conducted a retrospective analysis of all XEN45 gel stent implantations consecutively performed between March 2014 and June 2015 at the Department of Ophthalmology, Goethe University, Frankfurt, Germany [14]. For the present comparative analysis, we included the medical records of 148 eyes for which 12 months of data were available; 45 of these eyes were without prior glaucoma intervention and 103 eyes had failed prior glaucoma interventions. In general, patients presented with a moderate to advanced glaucoma stage. All procedures performed in studies involving human participants were in accordance with the ethical standards of the local Ethics Committee of the Goethe University, Frankfurt, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. According to the approval of the Ethics Committee of the Goethe University, Frankfurt, the data for this retrospective analysis were evaluated pseudonymized and no patient informed consent was required.
Patients and Assessments
Patients with refractory open-angle glaucoma (OAG) had been referred to our department from private practices with a request for surgical glaucoma therapy. Patients with an area of healthy, free, and mobile conjunctiva in the target quadrant and inadequately controlled IOP, optic disc damage, and progressive visual field loss despite maximum hypotensive topical therapy and/or prior filtering, cyclophotocoagulation, or laser interventions were considered suitable for XEN45 implantation. Exclusion criteria were angle-closure glaucoma, active neovascular glaucoma, prior conjunctival surgery, conjunctival scarring, or other conjunctival pathologies in the target quadrant, pregnancy, age < 18 years, condition after pars plana vitrectomy, flat anterior chamber and narrow chamber angle. Prior to XEN45 implantation a complete ophthalmic examination including gonioscopy had been performed. Postoperative evaluations were conducted at day 1, week 1, and months 1, 3, 6, and 12. At each visit, slit-lamp examination, gonioscopy, IOP assessment by Goldmann applanation tonometry (the mean value of three measurements per visit was recorded), as well as evaluation of the posterior pole were carried out. Moreover, the number of medications, needling, and adverse events were documented.
Surgical Technique
All implantations were performed by one single surgeon (FHH) following a standardized implantation technique which has been described in detail in our recent publication [14]. In brief, the XEN45 was implanted ab interno under peribulbar anesthesia. In order to prevent conjunctival scarring, 0.1 ml of MMC solution (0.01% mitomycin C, a total dose of 10 µg) was injected subconjunctivally into the nasal superior quadrant prior to XEN45 implantation. After a medium grade viscoelastic device was used to stabilize the anterior chamber, the preloaded injector needle was inserted through a 1.2-mm corneal paracentesis incision opposite the desired implantation site. The needle was then directed across the anterior chamber and the injector tip was used to penetrate through the chamber angle above the trabecular meshwork and the sclera at least 3 mm in length in order to place the implant properly. Viscoelastic substance was then thoroughly removed, paracenteses were hydrated, and the eye was covered with a patch.
Postoperatively, topical antibiotics were given four times daily for 10 days in combination with steroids six times daily and tapered out over 6 weeks. Antiglaucoma medication was given until surgery and was suspended on the day of surgery. In case of elevated IOP in the postoperative phase, additional antiglaucoma medication or secondary intervention was given at the discretion of the surgeon. In case of conjunctival scarring and bleb failure due to Tenon’s cyst formation, a needling procedure was performed under microscopic view in the operating room. The needling technique has recently been described in detail [14]. The administration of additional drugs during the needling was considered on a case-by-case basis. Only in cases of pronounced fibrosis 10 µg mitomycin C was injected during needling, while in eyes with cystic fibroses no additional drugs were used.
Statistical Analysis
For comparative analysis, the following two subgroups were defined: (a) eyes that had no prior glaucoma intervention but were on medication only, (b) eyes that had prior glaucoma interventions such as trabeculectomy, micro-bypass stent implantation, cyclophotocoagulation, laser procedures, and phacoemulsification. For more detailed analyses, group B was then further subdivided according to the various previous interventions.
Effectiveness variables were the success rates determined on the basis of the following definitions: “Complete success” was defined as an IOP reduction of at least 20% and an IOP value below 18 mmHg without medication. “Qualified success” was defined as an IOP reduction of at least 20% and an IOP value below 18 mmHg without or with medication. Additional effectiveness variables were mean IOP and the mean number of antiglaucoma medications, and their changes as compared to baseline, as well as the proportion of patients achieving a target IOP of ≤ 18 mmHg, ≤ 15 mmHg, and ≤ 13 mmHg at 12 months and needling rates. Safety outcomes included hypotony rate (IOP ≤ 6 mmHg) as well as intra- and postoperative complications. Data were presented as mean and standard deviation, unless otherwise indicated. Box plots of IOP data at 12 months were used to display the IOP distribution in both groups and to identify outliers. Baseline IOP was the IOP measured at the preoperative visit on medications. In order to calculate differences between pre- and postoperative values, the parametric t test was used. Also, the non-parametric Wilcoxon sign rank test was used for determining statistical significance within a group (p < 0.05 considered statistically significant). For determining statistical significance between both groups, t test for independent samples was applied (p < 0.05 considered statistically significant). Chi-square tests were performed in all other cases (p < 0.05 considered statistically significant).
Results
Overall, medical records of 148 eyes were included in this analysis. Of these, 45 eyes had no prior glaucoma intervention but were on medication only, while 103 eyes had failed a prior glaucoma intervention, including trabeculectomy (31 eyes), micro-bypass stent implantation (31 eyes), cyclophotocoagulation (24 eyes), phacoemulsification (11 eyes), and laser procedures (6 eyes). Within the group of eyes without prior glaucoma intervention, the XEN45 gel stent was implanted as standalone procedure in 32 eyes (71%) and in combination with phacoemulsification in 13 eyes (29%). Within the group of eyes with prior glaucoma interventions, 92 eyes (91%) received the XEN45 stent as standalone procedure and 9 eyes (9%) as combined procedure. For two eyes with prior glaucoma intervention, this information was missing.
Demographics and baseline ocular parameters of both groups (without/with prior glaucoma intervention) are displayed in Table 1. No statistically significant differences existed in demographic characteristics between the two groups (p > 0.05). Regarding baseline characteristics, eyes without prior intervention had a significantly higher mean baseline IOP than eyes with prior intervention (36.0 ± 10.7 mmHg vs. 31.6 ± 8.9 mmHg; p = 0.01; t test). Moreover, the mean number of hypotensive medications was significantly higher in eyes without prior intervention (3.6 ± 0.8 vs. 2.1 ± 1.0; p = 0.001; t test) (Table 1).
One year after XEN45 implantation, 76% (34/45) of eyes without prior intervention and 72% (74/103) of eyes with prior intervention achieved an IOP reduction of at least 20% and an IOP value below 18 mmHg with or without medication (qualified success). Complete success, i.e., an IOP reduction of at least 20% and an IOP value below 18 mmHg without medication, was experienced by 56% (25/45) of eyes without prior intervention and by 55% (57/103) of eyes with prior intervention 12 months after XEN45 implantation. There were no statistically significant differences between both groups regarding results of complete and qualified success (p = 0.994 and p = 0.665 respectively; chi-square test).
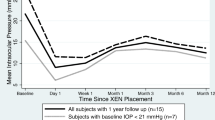
In both groups a significant reduction of the mean IOP was observed from the first postoperative day which lasted up to 1 year postoperatively. In eyes without prior glaucoma intervention, the mean IOP decreased significantly by 58% from preoperatively 36.0 ± 10.7 mmHg to 14.2 ± 3.4 mmHg at 12 months postoperatively (p = 0.000; Wilcoxon test), while in eyes with prior glaucoma intervention a mean IOP reduction by 53% from 31.6 ± 8.9 mmHg to 14.3 ± 4.2 mmHg was observed at 1 year postoperatively (p = 0.000; Wilcoxon test). No significant differences in mean IOP were found between the two groups at any visit during follow-up observation (p > 0.374 at each postoperative visit; t test) (Fig. 1). The box plots of the IOP at 12 months display a median IOP of 14 mmHg in both groups, with an interquartile range from 13 to 15 mmHg in eyes without prior glaucoma intervention and from 13 to 16 mmHg in eyes with prior glaucoma intervention (Fig. 2). With regard to the outliers, we carefully checked their medical history; however, no specific parameters could be identified that could explain the high IOP in these eyes.
Mean IOP at each study visit in eyes without prior interventional glaucoma therapy (n = 45) and eyes with prior interventional glaucoma therapy (n = 103). Error bars indicate SD for the mean. Within each group, the mean IOP was significantly reduced from baseline at any visit during the follow-up period (p = 0.000; Wilcoxon test). Preoperatively, significant differences were observed between both groups (p = 0.01; t test), while at each visit after gel stent implantation no significant differences were observed between groups regarding mean IOP (p > 0.374; t test)
Box plot of IOP at 12 months in eyes without prior glaucoma intervention (n = 45) and eyes with prior glaucoma intervention (n = 103), with 50% of IOP values (interquartile range) lying between 13 and 15 mmHg in eyes without prior glaucoma intervention and between 13 and 16 mmHg in eyes with prior glaucoma surgery
Also, the proportion of eyes achieving target pressure values of 18 mmHg, 15 mmHg, and 13 mmHg at 1 year postoperatively did not differ significantly between both groups (p > 0.05; chi-square test) (Fig. 3).
In both groups, the mean number of medications was significantly reduced at every visit from postoperative day one to the end of the 1-year follow-up period. Within eyes without prior glaucoma intervention, the mean number of antiglaucoma medications had significantly decreased from 3.6 ± 0.8 at baseline to 0.3 ± 0.7 medications at 12 months postoperatively (p = 0.000; Wilcoxon test), while in eyes with prior glaucoma intervention it had decreased from 3.0 ± 1.0 to 0.3 ± 0.7 medications (p = 0.000; Wilcoxon test). No significant differences between the groups were observed at any time during follow-up (p > 0.436 at each postoperative visit; t test) (Fig. 4).
Mean number of antiglaucoma medications at each study visit in eyes without prior glaucoma intervention (n = 45) and eyes with prior glaucoma intervention (n = 103). The mean number of medication was significantly reduced from baseline at all visits (p = 0.000; Wilcoxon test). Between groups, no significant differences were observed (p > 0.436; t test). Error bars indicate SD for the mean
While at baseline 60% (27/45) of eyes without prior glaucoma intervention required more than three medications, this was the case in 31% (32/103) of patients with prior glaucoma intervention. At 12 months all eyes without prior intervention and all but one eye with prior intervention needed less than three medications. Moreover, 78% (35/45) of eyes without prior glaucoma intervention and 83% (85/103) of eyes with prior glaucoma intervention were completely off drops 1 year postoperatively (Fig. 5).
Needling was required in 29% (13/45) of eyes with no prior glaucoma intervention and 35% (36/103) of eyes with prior glaucoma intervention within the 1-year follow-up period, with most procedures being performed within the early postoperative phase (between week 1 and month 3). The difference between both groups was not statistically significant (p = 0.57; chi-square test). Three eyes (6.7%) without prior glaucoma intervention and 8 eyes (7.8%) with prior glaucoma intervention presented with uncontrolled IOP during the follow-up phase. Together with the patients, it was decided to implant a second XEN gel stent in order to generate the possibility of additional aqueous humor drainage.
Hypotony (IOP ≤ 6 mmHg) was present in one eye without prior glaucoma intervention (2.2%) and in six eyes (5.8%) with prior glaucoma intervention at month 1 (p = 0.676; chi-square test). Within the group of eyes without prior glaucoma intervention, the single case of hypotony had resolved by month 3, while within the group of eyes with prior glaucoma intervention two cases of hypotony persisted until month 6. Both cases required filling of the anterior chamber and were resolved at 1 year. No macular folds, choroidal effusion or hemorrhage, choroidal detachment, or visual loss related to hypotony were observed. Moreover, no cases of device exposure or migration, corneal alterations, wound leakage, or endophthalmitis were observed in either group during the entire follow-up period. Complications or adverse events that were documented during the 1-year follow-up period are summarized in Table 2.
In order to further investigate if various previous glaucoma interventions may have different effects on the efficacy of a subsequent XEN gel implantation, we further subdivided the group of eyes with prior glaucoma interventions and analyzed mean IOP as well as mean number of medications at baseline and 12 months after XEN45 implantation. Moreover, we analyzed needling rates at 12 months postoperatively. Results are presented in Table 3. There was no statistically significant difference between the eyes without previous glaucoma intervention and eyes with the respective prior glaucoma interventions (p > 0.5; chi-square test).
Discussion
The aim of our retrospective analysis was to compare the efficacy of the XEN45 gel implant in patients with and without prior glaucoma intervention. Despite several studies demonstrating the efficacy and good safety profile of the XEN45 stent as initial surgical intervention [14, 16, 19, 20], there is not yet sufficient information available as to whether the XEN45 stent has comparable performance in patients who failed previous glaucoma interventions.
The results of our retrospective study show that patients with and without prior glaucoma intervention can benefit from the implantation of the XEN45gel stent. Both groups achieved an effective IOP and drug reduction that lasted until the end of the 1-year follow-up period. No significant differences were observed between the two groups. Overall, 76% of eyes without prior glaucoma intervention and 72% of the eyes with prior glaucoma intervention achieved qualified success (i.e., an IOP reduction of at least 20% and an IOP value below 18 mmHg with or without medication). In both groups, the mean IOP was effectively reduced from over 30 mmHg at baseline to under 15 mmHg at 12 months. Moreover, an IOP reduction of more than 50% was observed on the first day after XEN45 implantation and was sustained during the complete follow-up period in both groups. Also, the mean number of antiglaucoma medications was effectively reduced in both groups, from at least 3 to 0.3 medications, with more than three-quarters of eyes being completely off drops 1 year after XEN45 implantation in both groups. At the same time, hypotony was rarely observed in both groups. All cases of hypotony were completely resolved at 1 year and were not associated with complications threatening visual acuity. Also with regard to the requirement of a second filtrating implant or needling procedure, no significant differences between the two groups were found in our analysis. A subgroup analysis revealed that eyes with previous trabeculectomy had a tendency to have a higher needling rate at 12 months than eyes without prior glaucoma intervention (48% vs. 29%) or with any other prior intervention (Table 3). However, the differences were not statistically significant, which may be due to low case numbers.
In both groups, some eyes received the XEN45 gel implant in combination with phacoemulsification (21% of eyes without and 9% of eyes with prior glaucoma intervention). However, as shown in our analysis of the total population as well as in the preliminary results from the APEX study, the XEN45 gel stent results in a comparable and effective IOP reduction both as a sole procedure and in combination with phacoemulsification [14, 23]. It can therefore be assumed that the small number of combined procedures had no relevant influence on the results of the present comparison analysis.
Although we did not find any statistically significant differences, our data do provide some indications suggesting that eyes without prior glaucoma intervention might tend to respond slightly better to the XEN45 stent than pretreated eyes. Eyes without prior glaucoma treatment achieved a numerically higher success rate and greater reductions in IOP and antiglaucoma medication. At the same time, needling was numerically required less often in these eyes. Moreover at 12 months postoperatively, the IOP values in eyes with prior intervention indicated a slightly greater variance with a tendency towards higher IOP as compared to eyes without prior glaucoma intervention (Fig. 2). However, these assumptions must be treated with utmost caution, as no statistically significant differences were observed within 1-year follow-up within our analysis.
Overall, our data are in good agreement with the results of previously published retrospective and prospective studies, showing that the XEN45 gel stent is an effective method with a safe profile for controlling IOP in glaucoma patients. Furthermore, our results are consistent with the data of a recently published retrospective case series with 17 eyes receiving a XEN45 gel implant after failed trabeculectomy [22]. At month 12, the mean IOP was effectively reduced in these pretreated eyes from 21.5 mmHg preoperatively to 13.6 mmHg (p < 0.05) and at the same time medication was reduced from 2.8 preoperatively to 1.0 (p < 0.05). Hypotony (IOP < 6 mmHg) was observed in four cases that all resolved spontaneously, secondary filtration surgery was required in two cases, and postoperative bleb intervention was required in nine cases. Moreover, our data are also in line with the results from a post hoc data analysis of a prospective study presented during ASCRS 2018 [24]. In this analysis, 1-year results of the XEN45 stent in patients without (n = 24) and with (n = 41) and previous incisional glaucoma procedures were compared. It also revealed a similar effectiveness of the XEN45 stent regarding IOP and drug reduction in both groups. For instance, success rates were 83% in eyes without prior incisional surgery and 71% in eyes with prior incisional surgery, with success rate defined as proportion of patients achieving at least 20% IOP reduction on the same number or fewer medications as compared to baseline. Moreover, mean IOP was reduced from 25.7 to 14.9 mmHg in eyes without prior incisional surgery and from 24.8 mmHg at baseline to 16.6 mmHg at 12 months in eyes with prior incisional surgery. It is interesting to note that this analysis also found a trend towards greater reductions in IOP and antiglaucoma medication in eyes without prior incisional surgery; however, no statistically significant differences were observed. Taken together, these results demonstrate consistently that the XEN45 stent appears to be similarly effective in eyes without prior glaucoma intervention as well as in eyes with prior glaucoma intervention.
Our study has several limitations. First, it was a retrospective analysis of data from a single center, which might have led to bias. Nevertheless, this approach reflects the clinical routine and provides important insights into the efficiency of the XEN45 implant in clinical routine. Second, our results are limited to 12 months, which is a relatively short period in a lifetime of chronic disease. Thirdly, there was no control group, as both groups received the XEN45 implant, and finally the number of cases in our analysis may have been too small to detect statistically significant differences between the two groups.
Conclusion
The 1-year results of our retrospective analysis indicate that patients without and with previous glaucoma intervention can benefit from an implantation of the XEN45 gel stent. Reduction effects for IOP and antiglaucoma medication were similar in both groups, with a slight trend towards greater reductions in eyes without prior glaucoma intervention. Further controlled prospective studies with longer follow-ups are required to confirm our results.
References
Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081–90.
EGS. European Glaucoma Society guidelines, 4th ed. EGS; 2014. http://www.eugs.org. Accessed Nov 2018.
Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma. JAMA. 2014;311(18):1901–11.
Gedde SJ, Herndon LW, Brandt JD, et al. Postoperative complications in the tube versus trabeculectomy (TVT) study during five years of follow-up. Am J Ophthalmol. 2012;153:804–14.
Ansari E. An update on implants for minimally invasive glaucoma surgery (MIGS). Ophthalmol Ther. 2017;6:233–41.
Vera VI, Horvath C. XEN gel stent: the solution designed by Aquesys. In: Samples JR, Ahmed IIK, editors. Surgical innovations in glaucoma. New York: Springer; 2014. p. 189–98.
Lewis RA. Ab interno approach to the subconjunctival space using a collagen glaucoma stent. J Cat Refract Surg. 2014;40:1301–6.
Fellman RL, Feuer WJ, Grover DS. Episcleral venous fluid wave correlates with trabectome outcomes: intraoperative evaluation of the trabecular outflow pathway. Ophthalmology. 2015;122:2385–91.
Sheybani A, Dick B, Ahmed IIK. Early clinical results of a novel ab interno gel stent for the surgical treatment of open-angle glaucoma. J Glaucoma. 2016;25(7):e691–6.
Yu DY, Morgan WH, Sun X, et al. The critical role of the conjunctiva in glaucoma filtration surgery. Prog Retin Eye Res. 2009;28(5):303–28.
Sheybani A, Reitsamer H, Ahmed IIK. Fluid dynamics of a novel micro-fistula implant for the surgical treatment of glaucoma. Invest Ophthalmol Vis Sci. 2015;56:4789–95.
Directions for use for the XEN 45 GLAUCOMA TREATMENT SYSTEM. Europe
Directions for use for the XEN 45 GLAUCOMA TREATMENT SYSTEM. U.S.
Hengerer FH, Kohnen T, Mueller M, Conrad-Hengerer I. Ab interno gel implant for the treatment of glaucoma patients with or without prior glaucoma surgery—one year results. J Glaucoma. 2017;26:1130–6.
Grover DS, Flynn WJ, Bashford KP, et al. Performance and safety of a new ab interno gelatin stent in refractory glaucoma at 12 months. Am J Ophthalmol. 2017;183:25–36.
Galal A, Bilgic A, Eltanamly R, Osman A. XEN glaucoma implant with mitomycin C 1-year follow-up: result and complications. J Ophthalmol. 2017;2017:5457246.
De Gregorio A, Pedrotti E, Russo L, Morselli S. Minimally invasive combined glaucoma and cataract surgery: clinical results of the smallest ab interno gel stent. Int Ophthalmol. 2018;38:1129–34.
Sheybani A, Ahmed IK. Ab interno gelatin stent with mitomycin-C combined with cataract surgery for treatment of open-angle glaucoma: 1-year results. Presented at ASCRS 2015; 18 Apr 2015; San Diego, USA.
Pérez-Torregrosa VT, Olate-Pérez Á, Cerdà-Ibáñez M, et al. Combined phacoemulsification and XEN45 surgery from a temporal approach and 2 incisions. Arch Soc Esp Oftalmol. 2016;91:415–21.
Schlenker MB, Gulamhusein H, Conrad-Hengerer I, et al. Efficacy, safety, and risk factors for failure of standalone ab interno gelatin microstent implantation versus standalone trabeculectomy. Ophthalmology. 2017;124(11):1579–88.
Law SK, Shih K, Tran DH, Coleman A, Caprioli J. Long-term outcomes of repeat vs initial trabeculectomy in open-angle glaucoma. Am J Ophthalmol. 2009;148:685–95.
Karimi A, Hopes M, Martin KR, Lindfield D. Efficacy and safety of the ab-interno xen gel stent after failed trabeculectomy. J Glaucoma. 2018;27:864–8. https://doi.org/10.1097/IJG.0000000000001044.
Barton K, Sng C, VeraV. XEN45™ implantation for primary open-angle glaucoma: one-year results of a multicenter study. Poster presented at the ASCRS 2016 (P 6.01).
Sheybani A, Panarelli JF, Bashford KP, et al. Outcomes of ab interno gel stent placement with and without previous incisional glaucoma procedures. Presented at the ASCRS 2018, Washington DC, April 13–17.
Acknowledgements
Funding
No funding or sponsorship was received for this study. Article processing charges were funded by Allergan.
Editorial Assistance
Editorial support was funded by Allergan. The authors maintained complete control over the content of the paper. No payment was received for authorship of the document. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Fritz Hengerer, Ina Conrad-Hengerer, and Gerd Auffarth have nothing to disclose.
Compliance with Ethics Guidelines
All procedures performed in studies involving human participants were in accordance with the ethical standards of the local Ethics Committee of the Goethe University, Frankfurt, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. According to the approval of the Ethics Committee of the Goethe University, Frankfurt, the data for this retrospective analysis were evaluated pseudonymized and no patient informed consent was required.
Data Availability
AThe data sets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced digital features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.8224211.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License ( http://creativecommons.org/licenses/by-nc/4.0/ ), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hengerer, F.H., Auffarth, G. & Conrad-Hengerer, I. Comparison of Minimally Invasive XEN45 Gel Stent Implantation in Glaucoma Patients Without and With Prior Interventional Therapies. Ophthalmol Ther 8, 447–459 (2019). https://doi.org/10.1007/s40123-019-0193-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-019-0193-7