Abstract
Introduction
To determine anatomical, functional, and intraocular pressure (IOP) responses to diabetic macular edema (DME) treatments pre- and post-0.2 µg/day fluocinolone acetonide (FAc) implant administration compared with baseline and the preceding 3 years.
Methods
This was a retrospective, chart review, cohort study in four U.S. centers. Patients received the 0.2 µg/day FAc implant for the treatment of DME in at least one eye before January 1, 2016. DME treatments administered up to 36 months pre-FAc implant and up to 24 months post-FAc implant were recorded, and treatment frequency was calculated. Visual acuity (VA) was assessed using a Snellen eye chart and converted to early treatment diabetic retinopathy study (ETDRS) letters, and central subfield thickness (CST) was measured using optical coherence tomography (OCT). Treatment frequency, mean VA, mean CST, percentage of patients with CST of ≤ 300 µm, mean IOP, IOP events, and IOP treatments pre- and post-FAc implant administration were measured. Positive and negative predictive values for the IOP response to prior steroid therapy were also determined.
Results
In total, 160 eyes of 130 patients were studied. VA was maintained at pre-FAc levels from baseline to month 24, despite a significant reduction in treatment frequency from one treatment every 2.9 months pre-FAc implant to one treatment every 14.3 months post-FAc implant. Patients with better baseline VA required fewer DME treatments post-FAc than did patients with worse baseline VA. The majority of patients did not require additional DME treatment during the post-FAc follow-up period. A significant reduction in CST and an increase in the percentage of patients with CST of ≤ 300 µm were seen up to month 21 post-FAc implant. Pre-FAc implant IOP was maintained during the post-FAc implant period; increased IOP with prior steroid therapy was found to be highly predictive of increased IOP post-FAc implant.
Conclusion
The results of this study confirm the positive safety and efficacy profile of the FAc implant and demonstrate for the first time the effectiveness of the U.S. label indication of FAc in reducing the incidence of post-treatment pressure elevation. The FAc implant significantly reduced treatment burden in the overall population without significantly increasing the risk of steroid-induced pressure elevation.
Funding
Alimera Sciences.
Similar content being viewed by others
Introduction
Diabetic macular edema (DME) is a vision-limiting condition that is a consequence of diabetic retinopathy (DR) [1]. DME has been shown to be a multifactorial disease involving several inflammatory cytokines, particularly interleukin (IL)-1β, IL-6, IL-8, monocyte chemoattractant protein-1 (MCP-1), interferon-gamma-induced protein 10 (IP-10), and vascular endothelial growth factor (VEGF), all of which have been found in significantly higher concentrations in the aqueous humor of diabetic patients compared with non-diabetic patients [2, 3]. There are a number of treatment options for DME, of which the most commonly used are intravitreal anti-VEGF, intravitreal steroid treatment [4], and laser photocoagulation. Corticosteroids, such as triamcinolone, have been shown to reduce the levels of inflammatory cytokines—including VEGF—in the vitreous fluid [3]. The fluocinolone acetonide (FAc) implant (ILUVIEN®; Alimera Sciences Inc., Alpharetta, GA, USA) is a sustained-release intravitreal implant which releases a low dose (0.2 µg/day) of the corticosteroid FAc into the vitreous fluid over a period of 36 months [5]. The efficacy of the 0.2 µg/day FAc implant in the treatment of patients with DME has been demonstrated in the phase III Fluocinolone Acetonide for diabetic Macular Edema (FAME) study, which consisted of two randomized, sham injection-controlled, double-masked, multicenter clinical trials (FAME A and B) [6].
Increased intraocular pressure (IOP) and cataract formation are known adverse events associated with intravitreal corticosteroid therapy [7]. In the FAME study, no patients with prior steroid exposure and without a significant rise in IOP went on to require incisional glaucoma surgery [8]. Consequently, the 0.2 µg/day FAc implant was approved in the USA in 2014 for the treatment of DME in patients who have been previously treated with a course of corticosteroids without a clinically significant rise in IOP [9]. The real-world assessment of both the effectiveness and safety reported here is important since it is expected that treatment according to the U.S. indication will be associated with significantly less elevations in IOP compared with the FAME phase III clinical trial or other countries with different approval language.
The objectives of the US Retrospective Chart Review in Patients Receiving ILUVIEN (USER) study reported here were to determine anatomical and functional responses to DME treatments pre- and post-0.2 µg/day FAc implant administration and to compare these responses with baseline data in a large, real-world patient population who were treated according to the U.S. indication. The availability of detailed data up to 3 years prior to treatment also enabled a comparison of treatment frequency before and after 0.2 µg/day FAc implant administration as well as determination of the effect of 0.2 µg/day FAc implant administration on IOP and IOP events. In addition, unlike the FAME phase III clinical trial (which was conducted prior to the approval of anti-VEGF and dexamethasone pharmacotherapies for DME), we also assessed the effect of 0.2 µg/day FAc on patients transitioning from other DME therapies.
Methods
The USER study was a retrospective chart review of patients who received a 0.2 µg/day FAc implant for the treatment of DME in at least one eye before January 1, 2016. Four centers in the USA participated in the study: Cincinnati Eye Institute, Ohio; Georgia Retina, Georgia; Retina Health Institute, Florida; and Southern Eye, Mississippi. This study is an analysis of existing data and therefore constitutes a secondary use of the data requiring approval from an institutional review board—which was obtained—rather than individual patient consent.
We recorded DME treatments (anti-VEGF, steroid, or laser treatment) administered 36 months pre-FAc implant administration and up to 24 months post-FAc implant administration. We then calculated treatment frequency on a ‘mean number of treatments per month’ basis; we counted the number of DME treatments either pre- or post-FAc implant administration and divided these by the number of months of follow up pre- or post-FAc implant, respectively. The reciprocal of this number represents the average time interval between DME treatments. This approach normalized any differences between follow-up intervals pre- and post-FAc implant administration and allowed us to compare treatment frequency pre- and post-FAc treatment.
Visual acuity (VA) was assessed using a Snellen eye chart using the standard procedure for each site. Based on the method of assessment, we recorded VA as either uncorrected, pinhole, or best corrected. Results were recorded in Snellen fraction. For the purpose of performing statistical analysis, we converted VA values to approximate early treatment diabetic retinopathy study (ETDRS) letter scores based on the method described by Gregori et al. [10].
Spectral domain optical coherence tomography (OCT) was performed using either the Cirrus (Carl Zeiss Meditec, Jena, Germany) or the Heidelberg (Heidelberg Engineering, Heidelberg, Germany) OCT machines. We copied core data from each machine onto a portable hard drive and sent these data to Michael D. Abràmoff, MD, PhD at the Department of Ophthalmology and Visual Sciences, University of Iowa for analysis. Data obtained from the University of Iowa included the central subfield thickness (CST) and the macular volume.
We compared anatomical and functional measurements following treatment with the FAc implant, including mean CST, percentage of patients with CST of ≤ 300 µm, and mean VA in ETDRS letters, with pre-FAc treatment and baseline values (baseline was defined as the last assessment taken prior to FAc implant administration). We recorded mean IOP over the course of the study and IOP events (change in IOP from baseline, IOP elevations to ≥ 25 and ≥ 30 mmHg; incidence of trabeculectomy, trabeculoplasty, and incisional IOP-lowering surgery) pre- and post-FAc implant administration. We also noted the percentage of patients receiving IOP-lowering medications on the day of FAc implant administration and the percentage of patients who received IOP-lowering medication at any time following FAc implant administration.
In order to determine the IOP response that may occur with the FAc implant, we calculated positive and negative predictive values for the prior steroid. We used an IOP threshold of 25 mmHg with both prior steroid and the FAc implant to calculate the predictive values for both the maximum and last-recorded IOP following treatment with the FAc implant.
All eyes with any post-FAc follow up were included in the analysis. Differences from baseline were tested using a one-sample t test.
Results
Study Population
A total of 160 eyes of 130 patients were included in the study. Baseline characteristics and demographics are shown in Table 1. Prior steroid treatments included difluprednate, prednisolone acetate, triamconolone acetonide, and dexamethasone.
VA Outcomes and Treatment Frequency
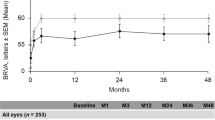
In the entire population, VA was maintained at pre-FAc administration levels from baseline to month 24 following FAc implant administration (Fig. 1).
Most (91.3%) eyes were receiving therapy prior to FAc implant administration (Table 1). Of the 146 eyes which had received prior therapy, the majority (84.2%) had received prior therapy with anti-VEGF, 61.6% had received prior intravitreal steroid treatment (triamcinolone or dexamethasone), and 54.8% had received prior laser treatment. Following FAc treatment, 63% of eyes did not require additional DME treatments up to month 24. Of the 60 patients who required treatment for DME following FAc implant administration, 74.6% of the treatments were anti-VEGF therapy, 14.9% of the treatments were intravitreal steroids and 10.4% of the treatments were laser.
There was a significant reduction in treatment frequency following FAc implant administration in the full-study population of 160 eyes. One treatment was administered every 2.9 months pre-FAc implant administration, which declined to one treatment every 14.3 months post-FAc implant administration (P < 0.001).
We also examined VA outcomes and treatment frequencies by baseline VA category on the day of treatment with the FAc implant (Fig. 2). The reductions in treatment burden were clinically relevant and statistically significant for all subgroups analyzed. Eyes with better baseline VA scores were treated less frequently post-FAc administration than eyes with poorer baseline VA, and this reduction of treatment burden post-FAc occurred in a ‘dose response-type’ fashion with regards to baseline VA. The subgroup of patients with the greatest reduction in treatment frequency was the group with the best baseline VA (≥ 20/40). VA was maintained post-FAc administration with a significantly reduced treatment frequency, from one treatment every 2.9 months to one every 22.0 months (P < 0.001). This trend continued in the intermediate subgroups. In the subgroup with VA < 20/40–20/100, treatment frequency was reduced from one treatment every 3.2 months to one treatment every 15.2 months (P < 0.001), and the VA remained stable (P = 0.771). In the subgroup with VA < 20/100–20/200, treatment frequency was reduced from one treatment every 2.3 months to one treatment every 7.0 months (P < 0.001) and the VA improved, although this difference was not statistically significant (P = 0.136). In the subgroup of patients with the worst baseline VA (< 20/200), treatment frequency was reduced from one treatment every 2.9 months to one treatment every 6.7 months (P = 0.026). In this latter subgroup with poor vision, post-FAc VA improved significantly (P = 0.022) compared to baseline.
Visual acuity (VA) pre- and post-0.2 µg/day fluocinolone acetonide (FAc) implant administration by baseline VA in 160 eyes. Asterisk (*) indicates significant difference in VA between pre- and post-0.2 µg/day FAc implant administration at P < 0.001; dagger (†) indicates significant difference in VA between pre- and post-0.2 µg/day FAc implant administration at P = 0.026
Retinal Thickness Outcomes
In the study population for whom we could directly download the machine source OCT data from the machine for analysis (n = 120 eyes), we observed a reduction in CST following FAc implant administration, which was significant through 21 months post-FAc administration, following which the reduction in CST trended towards significance at 24 months (P = 0.089; n = 6) (Fig. 3a). There was a significant increase in the percentage of patients with CST of ≤ 300 µm following FAc implant administration (Fig. 3b). At month 18 pre-FAc implant administration, 35.4% of patients had CST of ≤ 300 µm; at month 18 post-FAc administration, 60.0% of patients had CST of ≤ 300 µm.
Central subfield thickness (CST) data for the optical coherence tomography (OCT) population (n = 120). a Mean CST pre- and post-0.2 µg/day FAc implant administration, b percentage of eyes with CST of ≤ 300 μm. P values represent significance of change in CST from baseline. Numbers above time points indicate the number of eyes for which data were available at that time point
IOP Outcomes
The IOP was maintained following FAc implant administration (Fig. 4). There was a numerical, but not a statistically significant reduction in the incidence of IOP-related events following FAc implant administration (Table 2). Incidences of trabeculoplasty and incisional IOP-lowering surgery were similar post-FAc implant administration compared with pre-administration (1.3 vs. 1.9% and 1.3 vs. 1.3%, respectively).
In the USER population, the mean follow-up was approximately 12 months post-FAc implant administration. When we compared the data at month 12 between the USER population and the phase III FAME study, the mitigating effect of the Food and Drug Administration indication requiring prior steroid challenge was evident, particularly in the prevalence of all IOP events, with the exception of IOP-lowering medication use (Table 3).
Predictability of IOP Elevation Associated with the 0.2 µg/day FAc Implant Based on IOP Following Prior Steroid Use
A lack of prior clinically significant IOP elevation following steroid use had a very high positive predictive value for a lack of IOP elevation post-FAc implant administration: 98 and 94% using the last IOP and maximum IOP, respectively (Table 4). That is, if an IOP of ≤ 25 mmHg is maintained on the prior steroid therapy, there is a high probability that an IOP of ≤ 25 mmHg will also be maintained during treatment with the FAc implant.
Discussion
The USER study demonstrates the value of continuous microdosing of the FAc implant to improve physician control over patients’ DME without significantly increasing IOP. VA was maintained or improved in patients following FAc implant administration, with an 80% reduction in treatment frequency in the full population.
Continuous delivery of corticosteroids to the posterior segment of the eye is only possible if extremely low doses of drug are released. Such low doses of corticosteroid are only clinically effective if the steroid is highly lipophilic and penetrates the retina very easily. FAc is the most lipophilic corticosteroid approved for the treatment of DME. This difference in lipophilicity can be quantified by the water solubility of FAc (0.0547 mg/ml) [11] versus dexamethasone (0.1 mg/ml) [12], and by the differential distribution of these molecules in the vitreous fluid versus the retina seen in animal studies assessing drug distribution, where FAc preferentially accumulated in the retina and dexamethasone preferentially accumulated in the vitreous fluid [13, 14]. Thus, FAc is ideally suited for the type of long-term delivery provided by the FAc implant.
All baseline VA subgroups experienced a significant reduction in the number of DME treatments needed in the setting of maintained or improved VA following treatment with the FAc implant. Patients with better VA at the time of FAc implant administration experienced the greatest reductions in treatment frequency (87%), while maintaining VA. Patients with the poorest VA at the time of FAc implant administration experienced an improvement in VA, alongside a 57% reduction in treatment frequency.
Prior to FAc implant administration, patients received approximately one treatment every 3 months, regardless of VA. This frequency is lower than treatment rates studied in randomized controlled trials [15] but is similar to previously published ‘real-world’ data from large commercial databases [16, 17]. The important and real differences between trial populations and real-world patients are well known. Following FAc implant administration in the USER study, all eyes received fewer treatments. Patients with poorer vision at baseline (< 20/200) received adjuvant treatments more frequently (one treatment every 6.7 months) than did those with better vision at baseline (≥ 20/40, one treatment every 22.0 months). Following treatment with the FAc implant, 63% of eyes did not require further DME treatment, regardless of vision.
There was a reduction in CST following FAc implant administration in the overall study population analyzed with OCT. The mean CST improved from 370.4 to 276.6 µm after treatment with the FAc implant. There was also a significant increase from 28.2 to 83.3% in the percentage of patients who achieved ≤ 300 µm CST post-FAc implant administration (Fig. 3b).
In FAME, a larger proportion of IOP events were reported post-FAc administration compared with the results of the USER study (Table 3). Interestingly, IOP events in USER were similar pre- and post-FAc implant administration, indicating that FAc did not cause an increase in the incidence of IOP events above that seen with prior steroid treatment in the USER study population. All patients enrolled in the USER study had received a prior course of corticosteroids without a clinically significant rise in IOP, in line with the U.S. label requirements; [9] this was not a requirement in the FAME study. It seems likely that this difference is responsible for the marked reduction in IOP events seen in this real-world patient population.
The positive predictability of a lack of IOP response observed with the prior steroid in determining the likelihood of a lack of IOP response (94.2–98.1%) following the FAc implant is high. Therefore, the risk of an uncontrolled IOP response post-FAc implant is low in patients who have not experienced a clinically significant increase in IOP with prior steroid treatments. These USER study outcomes support the effectiveness of the US indication in successfully mitigating the potential risk of IOP increases associated with the administration of the FAc implant.
All approved therapies for DME other than FAc require regular repeated treatments to maintain a therapeutic effect. The FAc implant creates a therapeutic foundation with one administration. This was adequate for most patients in our cohort to treat and control their DME, but also allowed for the addition of intermittent adjunctive treatment when needed. The FAc implant therefore reduces treatment burden regardless of vision and provides a unique and valuable treatment option for DME patients.
The USER study was a retrospective chart review in which each patient’s treatment and disease history prior to FAc treatment served as the control. As such, confounding factors and the absence of a parallel comparator arm may limit interpretations of efficacy compared with a randomized, prospective clinical trial. In addition, the analysis is based on U.S. data and might not be generalizable to a broader population or different healthcare systems. Nevertheless, this comparison of pre- and post-FAc data in a real-world setting shows that treatment burden and CST are reduced and that VA is maintained or improved following FAc implant administration.
Conclusions
Overall, the USER study found the FAc implant to be effective in reducing edema and decreasing treatment frequency. This is uniquely achieved with mitigation of IOP risk due to the specific properties of FAc, the continuous microdosing achieved with the use of the implant, and by adherence to the U.S. label requirement of prior steroid use without a clinically significant IOP elevation.
References
Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–64.
Dong N, Xu B, Wang B, Chu L. Study of 27 aqueous humor cytokines in patients with type 2 diabetes with or without retinopathy. Mol Vis. 2013;19:1734–46.
Sohn HJ, Han DH, Kim IT, Oh IK, Kim KH, Lee DY, et al. Changes in aqueous concentrations of various cytokines after intravitreal triamcinolone versus bevacizumab for diabetic macular edema. Am J Ophthalmol. 2011;152:686–94.
Wenick A, Bressler N. Diabetic macular edema: current and emerging therapies. Middle East Afr J Ophthalmol. 2012;19:4–12.
Campochiaro PA, Brown DM, Pearson A, Ciulla T, Boyer D, Holz FG, et al. Long-term benefit of sustained-delivery fluocinolone acetonide vitreous inserts for diabetic macular edema. Ophthalmology. 2011;118:626–35.
Campochiaro PA, Brown DM, Pearson A, Chen S, Boyer D, Ruiz-Moreno J, et al. Sustained delivery fluocinolone acetonide vitreous inserts provide benefit for at least 3 years in patients with diabetic macular edema. Ophthalmology. 2012;119:2125–32.
Reichle ML. Complications of intravitreal steroid injections. Optometry. 2005;76:450–60.
Parrish RK, Campochiaro PA, Pearson PA, Green K, Traverso CE. Characterization of intraocular pressure increase and management strategies following treatment with fluocinolone acetonide intravitreal implants in the FAME trials. Ophthalmic Surg Lasers Imaging Retina. 2016;47:426–35.
Alimera Sciences Limited. ILUVIEN® prescribing information. http://www.alimerasciences.com/wp-content/uploads/2016/02/Prescribing-Information.pdf. Accessed 8 Mar 2018.
Gregori NZ, Feuer W, Rosenfeld PJ. Novel method for analyzing Snellen visual acuity measurements. Retina. 2010;30:1046–50.
DrugBank. Fluocinolone acetonide. https://www.drugbank.ca/drugs/DB00591. Accessed 13 Mar 2008.
O’Neill MJ. The Merck index: an encyclopedia of chemicals, drugs, and biologicals. 13th ed. Whitehouse Station, NJ: Merck; 2001. p. 518.
Kane FE, Green KE. Ocular pharmacokinetics of fluocinolone acetonide following Iluvien implantation in the vitreous humor of rabbits. J Ocul Pharmacol Ther. 2015;31:11–6.
Chang-Lin JE, Attar M, Acheampong AA, Robinson MR, Whitcup SM, Kuppermann BD, et al. Pharmacokinetics and pharmacodynamics of a sustained-release dexamethasone intravitreal implant. Investig Ophthalmol Vis Sci. 2011;52:80–6.
Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, Feiner L, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119:789–801.
Kiss S, Liu Y, Brown J, Holekamp NM, Almony A, Campbell J, et al. Clinical utilization of anti-vascular endothelial growth-factor agents and patient monitoring in retinal vein occlusion and diabetic macular edema. Clin Ophthalmol. 2014;8:1611–21.
Kiss S, Chandwani HS, Cole AL, Patel VD, Lunacsek OE, Dugel PU. Comorbidity and health care visit burden in working-age commercially insured patients with diabetic macular edema. Clin Ophthalmol. 2016;10:2443–53.
Acknowledgements
The authors would like to thank all the participants of the USER study.
Funding
Sponsorship for this study and article processing charges were funded by Alimera Sciences. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Medical Writing, Editorial, and Other Assistance
Editorial assistance in the preparation of this article was provided by Emma Mycroft, PhD of Helios Medical Communications, Alderley Edge Cheshire, UK. Support for this assistance was funded by Alimera Sciences. Biostatistical analyses were provided by Alimera Sciences. Hussein Wafapoor, MD, Fort Myers, Florida was an investigator in the USER study. OCT data analysis was provided by Michael D Abràmoff, MD, PhD, Iowa City, Iowa.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Alexander Eaton reports a relationship with Alimera Sciences during the conduct of the study and personal fees from Alimera Sciences outside the submitted work; he also reports stock ownership in Alimera Sciences, Psivida, and Regeneron. Sean S. Koh reports a relationship with Alimera Sciences during the conduct of the study and personal fees from Alimera Sciences outside the submitted work. Jaime Jiminez reports personal fees and other from Alimera Sciences Ltd. during the conduct of the study. Christopher D. Riemann reports that he is a consultant for Alcon, Alimera, HumanOptics AG, Haag Streit USA, Gore, Haag Streit Surgical, Haag Streit AG, Iamc2, Janssen/Johnson & Johnson, Kaleidoscope Engineering, MedOne, NotalVision LLC, Reliance Industries, Salutaris MD, and TrueVision; has received research funding from Aerpio, Alcon, Clearside Biomedical, Genentech, Janssen/Johnson & Johnson, and Regeneron; has presented for Alcon, Alimera, Allergan, Reliance Industries, Salutaris MD; is the owner or cofounder of Chruman Research, Macor Industries, Northmark Pharmacy, and VEO; has intellectual property with Haag Streit AG, Iamc2, Janssen/Johnson & Johnson, MedOne, Reliance Industries, and Vortex Surgical; and has other financial relationships with MedOne, Reliance Industries, TrueVision, and Vortex Surgical.
Compliance with Ethics Guidelines
This study is an analysis of existing data and therefore constitutes a secondary use of the data requiring approval from an institutional review board—which was obtained—rather than individual patient consent.
Data Availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced digital features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.7352066.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Eaton, A., Koh, S.S., Jimenez, J. et al. The USER Study: A Chart Review of Patients Receiving a 0.2 µg/day Fluocinolone Acetonide Implant for Diabetic Macular Edema. Ophthalmol Ther 8, 51–62 (2019). https://doi.org/10.1007/s40123-018-0155-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-018-0155-5