Abstract
Introduction
To determine the incidence of ischemic cerebral stroke in the 6-month periods preceding and following acute central retinal artery occlusion (CRAO) among Medicare beneficiaries.
Methods
A retrospective cohort study with comparison group conducted for calendar year 2013. Patients with CRAO were identified through National Medicare limited inpatient and institutional outpatient datasets for emergency services using ICD-9-CM code for CRAO (362.31). Patients with hip fractures (ICD-9-CM 820–820.9) during the same time period served as controls. Interval incident rates of ischemic stroke were determined from time-coded diagnoses of CRAO and hip fracture (index date zero) to date of principal discharge diagnosis of ischemic stroke (ICD-9-CM 434) recorded in the Medicare inpatient dataset. Risk of stroke was examined by comparing incidence among the two cohorts preceding and following the sentinel events.
Results
There were 3338 patients with CRAOs during 2013. The incidence of ischemic stroke peaked the second week following CRAO relative to patients with hip fracture (relative incidence = 33.1 [95% confidence interval 9.8–84.6]).
Conclusions
Medicare beneficiaries who present to emergency rooms with CRAO or are hospitalized directly for this condition were at highest risk of ischemic stroke in the first 2 weeks following the ocular diagnosis. Patients with acute CRAO should be promptly evaluated for stroke and stroke prevention.
Similar content being viewed by others
Introduction
The increased risk of ischemic strokes in persons with central retinal artery occlusion (CRAO) has been demonstrated in several retrospective studies [1,2,3,4,5]. The association is biologically feasible on the basis of shared risks factors for stroke and CRAO, and the known pathogeneses of atherosclerosis and thromboembolic diseases [6,7,8]. Understanding the temporal risk of ischemic stroke in the setting of recent CRAO is critical for appropriate clinical management, since there are effective methods for stroke prevention if instituted in a timely manner [9]. Only one previous study has examined the risk of stroke in the time immediately surrounding CRAO [2]. In that self-controlled case series, the authors found that the relative incidence rate ratio for ischemic stroke among patients with CRAO compared to the age- and gender-matched Korean population was roughly 70 times greater during the week immediately following the ocular event. Although an increased risk of this magnitude has important clinical implications, the temporal risk in stroke has not been studied in the USA. As risk profiles for stroke vary widely throughout regions of the world, documenting the temporal relationship between CRAO and ischemic stroke in a population from the USA is needed [10]. We examined the incidence of ischemic stroke in the weeks and months before and following CRAO among Medicare beneficiaries in the USA, using a control group with hip fracture.
Methods
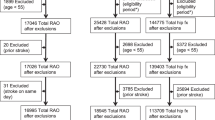
We employed the national Medicare limited inpatient and institutional outpatient datasets for calendar years 2011–2014 to identify patients through ICD-9-CM code for CRAO and ischemic stroke for year 2013. The codes are linkable across datasets through a scrambled patient identifier, with online documentation [11]. Patients with ICD-9-CM codes for CRAO (362.31) during 2011–2012, and ischemic stroke (434) in 2011 and first half of 2012 were excluded. Patients with CRAO in 2013 were identified using both Medicare inpatient and institutional outpatient datasets for emergency department services. The date of diagnosis of CRAO was used as the index date for each case, so that look-back and look-forward periods of 180 days for individual patients could be determined. The Medicare inpatient dataset for ICD-9-CM code 434 entered during 180 days preceding and following index dates were searched and linked longitudinally with a scrambled patient identifier. The number of new ischemic strokes among Medicare beneficiaries per designated time periods was derived from the principal diagnosis for stroke (ICD-9-CM 434) after excluding patients with previous CRAO and stroke during the look-back period.
The control group consisted of patients diagnosed with new hip fracture (principal diagnosis ICD-9-CM 820–820.9) in 2013. We excluded patients with previous hip fracture in 2012 and stroke outside the 180 days prior to the hip fracture index date, in keeping with the same exclusionary period for the CRAO patient group. Medically relevant comorbidities for both groups were identified through ICD-9-CM codes for diabetes (ICD-9-CM 250–259), systemic hypertension (ICD-9-CM 401.0, 401.1, 401.9), hyperlipidemia (ICD-9-CM 272), smoking (ICD-9-CM 305.1), congestive heart failure (ICD-9-CM 428), renal failure (ICD-9-CM 586), chronic respiratory disease (ICD-9-CM 490–496), peripheral vascular disease (ICD-9-CM 443.9), and atrial fibrillation (ICD-9-CM 427.31). Given the general difficulty in designating an ideal control group, patients with hip fracture were chosen as they are under close medical supervision so that a diagnosis of stroke would unlikely be delayed or overlooked, even if minor. We also wanted a control group that was at relatively high risk of new stroke so any bias in relative risk would be towards the null [12].
Statistical analysis
The Chi-square test was used to determine whether no differences in observed proportions of comorbidities existed between study groups (α = 0.01). Incident rates were determined for time intervals both before and after the sentinel events of CRAO and hip fracture similar to that chosen by Park et al. [2]. The incidence of new strokes was determined for the following time intervals after the index date of CRAO and hip fracture: first week; second week; third and fourth weeks, then 30-day intervals until 6 months. The same time intervals were used to describe the stroke rates prior to sentinel events. Results were adjusted for age and gender on the basis of the overall 2013 Medicare population. The 95% confidence interval for incidence was calculated for each time interval using a Poisson distribution [13]. Analyses used SAS®, version 9.4 Cary, NC. The Northwestern University Institutional Review Board granted a study exemption. This article does not contain any studies with human participants or animals performed by any of the authors.
Results
After exclusion criteria were applied, 3338 CRAOs (ICD-9-CM 362.31) and 187,999 hip fractures (ICD-9-CM 820–820.9) were identified among 26,275,521 Medicare beneficiaries in 2013. The 5-year interval age distribution of patients with CRAO was < 65, 9.8%; 65–69, 16.5%; 70–74, 18.8%; 75–79, 18.3%; 80–84, 17.4%; > 84, 19.2%. Women made up 47.7% of the cohort. The 5-year interval age distribution for the control group was < 65, 4.6%; 65–69, 6.8%; 70–74, 9.2%; 75–79, 13.3%; 80–84, 20.1%; > 84, 46.0%. Women comprised 71.3% of the control group. Baseline medical comorbidities among patients with CRAO and hip fracture, and the general Medicare population are shown in Table 1. There were statistically significant differences in the proportions of all comorbidities among the groups. Both the CRAO and hip fracture groups had substantially increased proportions of key risk factors for stroke, including high blood pressure, diabetes mellitus, smoking, and atrial fibrillation (Table 1).
In the 6 months preceding CRAO, 49 patients were diagnosed with ischemic stroke. This number rose to 141, during the 6 months following CRAO. Most ischemic strokes occurred during the 2 months that preceded and followed CRAO, when a total of 14 strokes occurred 30 days before the ocular event and 91 strokes 30 days after the ocular event. Among patients with hip fractures, there were 2945 with strokes, of which 1551 occurred 6 months before and 1394 occurred 6 months after hip fracture. The number of strokes occurring 30 days before and 30 days after hip fracture was 282 and 344, respectively.
Table 2 shows the age- and gender-adjusted incidence of strokes among patients with CRAOs and hip fracture for eight time intervals before and after the event dates. The incidence of stroke in the CRAO cohort compared to those with hip fracture was significantly elevated during the first week (27.6 [95% CI 7.3–69.9]) and second week (33.1 [95% CI 9.8–84.6]) following the sentinel events (Fig. 1).
Discussion
This study found a 28-fold and 33-fold increase in incidence of ischemic stroke in the first and second weeks following CRAO compared to the group with hip fracture. This finding is consistent with that reported by Park et al., who found a 70-fold increase in ischemic stroke the first week after CRAO and a near 22-fold increase within 3 days [2]. The lower rates of stroke found in our study are not unexpected as the study by Park et al. did not have a designated control group for comparison. Persons with hip fracture tend to have high levels of other medical comorbidities, including stroke. Stroke is also a risk factor for hip fracture [12, 14, 15].
The clinical implications of our findings are important as patients who present with recent CRAO are at substantial risk of ischemic stroke within a brief time frame after the ocular event. The American Academy of Ophthalmology’s Preferred Practice Pattern recommends that patients with acute CRAO be promptly referred for stroke evaluation and prevention [16]. Clinical practice patterns for the management of CRAO, however, vary considerably depending on physician training and background. In a survey published in 2009, only 35% of ophthalmologists refer a patient with acute CRAO to the emergency department for immediate evaluation compared to 73% of neurologists and 86% of neuro-ophthalmologists [17].
Our study has several potential limitations. First, this study used administrative datasets. Goldstein noted in Medicare claims data that about 20% of patients with acute ischemic stroke had other conditions [18]. More recent studies of administrative datasets, however, have demonstrated more reliable coding [19]. Given the striking peak in relative incidence after CRAO, even if a quarter of the diagnoses were miscoded, this would still leave a sizable increase in stroke incidence. In addition, miscoding of stroke should not occur preferentially among patients with CRAO. Thus, the relative differences between the study and comparison groups should be unaffected.
Second, persons hospitalized to exclude stroke may be more likely to undergo multiple diagnostic procedures including diffusion-weighted magnetic resonance imaging, an increasingly sensitive method of detecting ischemic stroke, than persons evaluated as outpatients [1, 20, 21]. The sensitivity of diffusion-weighted imaging in detecting small, often asymptomatic infarcts in the cerebral hemispheres may increase the diagnosis of ischemic stroke beyond the perceived risk of stroke following CRAO based on older technologies [21]. Although this increase in diagnostic sensitivity may be clinically beneficial, it also explains why previous perceptions of stroke risk were less.
Third, patients who are hospitalized after CRAO may represent a subset of all patients with CRAO who have stronger or more clinically obvious predictors of stroke such as atrial fibrillation. This type of selection bias could overestimate the true incidence of ischemic stroke among all patients with CRAO.
Although incidence of stroke was standardized according to age and gender to reduce distortions of rates between the CRAO study group and those with hip fracture, it was not possible to adjust rates for major risk factors of stroke as patient-specific medical diagnoses could not be linked after patients were segregated into time intervals. Over two-thirds of patients with CRAO also have undiagnosed cardiovascular risk factors for stroke at the time of their ocular event [22]. Thus knowledge of critical risk factors changes substantially after patients present with vision loss.
Conclusions
Based on findings from administrative datasets, the relative incidence of ischemic stroke peaks abruptly 2 weeks following CRAO. The results are consistent with results from the only previous time-dependent study in the literature [2]. These findings underscore the importance of promptly referring patients with acute CRAO to stroke centers or stroke-ready hospitals for further evaluation [16].
References
Chang YS, Jan RL, Weng SF, et al. Retinal artery occlusion and the 3-year risk of stroke in Taiwan: a nationwide population-based study. Am J Ophthalmol. 2012;154(4):645–52.
Park SJ, Choi N-K, Yang BR, et al. Risk and risk periods for stroke and acute myocardial infarction in patients with central retinal artery occlusion. Ophthalmology. 2015;122(11):2336–43.
Hyungtaek T, Jan J, Choi YS, et al. Retinal artery occlusion and the risk of stroke development. Twelve-year nationwide cohort study. Stroke. 2016;47(2):376–82.
Christiansen DB, Lip GYH, Lamberts M, et al. Retinal vein and artery occlusions: a risk factor for stroke in atrial fibrillation. J Thromb Haemost. 2013;118:1485–92.
Ueda Y, Kanazawa S, Ohira A, et al. Retinal vascular obstruction and asymptomatic cerebral infarction. Jpn J Ophthalmol. 2002;462:209–14.
Hayreh SS, Podhajsky PA, Zimmerman MB. Retinal artery occlusion: associated systemic and ophthalmic abnormalities. Ophthalmology. 2009;116:1928–36.
Park SJ, Choi NK, Seo KH, Park KH, Woo JS. Nationwide incidence of clinically diagnosed central retinal artery occlusion. Ophthalmology. 2014;121(10):1933–8.
Callizo J, Feltgen N, Pantenburg S, et al. Cardiovascular risk factors in central retinal artery occlusion: results of a prospective and standardized medical examination. Ophthalmology. 2015;122(9):1881–8.
Ezekowitz JA, Straus SE, Majumdar SR, McAlister FA. Stroke: strategies for primary prevention. Am Fam Physician. 2003;68(12):2379–86.
Goto S, Ikeda Y, Chan JCN, et al. Risk-factor profile, drug usage and cardiovascular events within a year in patients with and at high risk of atherothrombosis recruited from Asia as compared with those recruited from non-Asian regions: a substudy of the Reduction of Atherothrombosis for Continued Health (REACH) registry. Heart Asia. 2011. https://doi.org/10.1136/ha.2010.002691.
Research Data Assistance Center. http://www.resdac.org/cms-data/file-family/LDS-Medicare-Claims. Accessed 13 Mar 2017.
French DD, Bass E, Bradham DD, Campbell RR, Rubenstein LZ. Rehospitalization after hip fracture: predictors and prognosis from a national Veterans study. J Am Geriatr Soc. 2008;56(4):705–10.
Rothman KJ, Greenland S. Modern epidemiology. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2008.
Ramnemark A, Nilsson M, Borssén B, Gustafson Y. Stroke, a major and increase risk factor for femoral neck fracture. Stroke. 2000;31(7):1572–7.
Yuan ZC, Mo HL, Guan J, et al. Risk of hip fracture following stroke, a meta-analysis of 13 cohort studies. Osteoopros Int. 2016;27(9):2673–9.
Retinal and Ophthalmic Artery Occlusions Preferred Practice Pattern. American Academy of Ophthalmology®. 2016. https://www.aao.org/Assets/c0e05d81-529f-4273-b4a1-66a06e0f6fcb/636217289308000000/retinal-and-artery-ophthalmic-artery-occlusions-ppp-pdf. Accessed 15 May 2017.
Atkins EJ, Bruce BB, Newman NJ, Biousse VA. Translation of clinical studies to clinical practice: survey on the treatment of central retinal artery occlusion. Am J Ophthalmol. 2009;148(1):172–3.
Goldstein LB. Accuracy of ICD-9-CM coding for the identification of patients with acute ischemic stroke. Effect of modifier codes. Stroke. 1998;29:1602–4.
Porter J, Mondor L, Kapral MK, Fang J, Hall RE. How reliable are administrative data for capture stroke patients and their care? Cerebrovasc Dis Extra. 2016;6(3):96–106.
Helenius J, Arsava EM, Goldstein JN, et al. Concurrent acute brain infarcts in patients with monocular visual loss. Ann Neurol. 2012;72(2):286–93.
Lee J, Kim SW, Lee SC, Kwon OW, Kim YD, Byeon SH. Co-occurrence of acute retinal artery occlusion and acute ischemic stroke: diffusion-weighted magnetic resonance imaging study. Am J Ophthalmol. 2014;157(6):1231–8.
Callizo J, Feltgen N, Pantenburg S, et al. European Assessment Group for Lysis in the Eye. Cardiovascular risk factors in central retinal artery occlusion: results of a prospective standardized medical examination. Ophthalmology. 2015;122:1881–8.
Acknowledgements
We would like to thank Karl Y. Bilimoria, MD, MS, Director of the Surgical Outcomes Quality Improvement Center (http://www.soqic.org), for his assistance in obtaining the Medicare data. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government (French, Greenberg).
Funding
Dr. French is supported by an unrestricted grant from Research to Prevent Blindness, New York, NY and Department of Health and Human Services National Institutes of Health, NATIONAL EYE INSTITUTE Grant number: 1R21EY024050-01A1. Research to Prevent Blindness, New York, NY supported the design and conduct of the study; data collection, and management. No funding was received for the article processing charges.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author contributions
Category 1 (a) Concept and design: all authors; (b) acquisition of data: DF; (c) analysis and interpretation of data: all authors. Category 2 (a) drafting of manuscript: CM; (b) revising it for intellectual content: all authors. Category 3 (a) final approval: all authors.
Compliance with ethics guidelines
The Northwestern University Institutional Review Board granted a study exemption. This article does not contain any studies with human participants or animals performed by any of the authors.
Disclosures
Dustin D. French, Curtis E. Margo, and Paul B. Greenberg have nothing to disclose.
Data availability
Medicare Limited Data Set (LDS) files contain beneficiary level protected health information and are under Federal regulation through the Health Insurance Portability and Accountability Act; By law, LDS requests require a Data Use Agreement (DUA) available at https://www.resdac.org/cms-data/request/limited-data-sets and require an Institutional Board Waiver. LDS requests do not require a ResDAC review and can be submitted directly to CMS by the researcher. For further information visit https://www.resdac.org/.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced content
To view enhanced content for this article go to https://doi.org/10.6084/m9.figshare.5982250.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
French, D.D., Margo, C.E. & Greenberg, P.B. Ischemic Stroke Risk in Medicare Beneficiaries with Central Retinal Artery Occlusion: A Retrospective Cohort Study. Ophthalmol Ther 7, 125–131 (2018). https://doi.org/10.1007/s40123-018-0126-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40123-018-0126-x