Abstract
Introduction
Rotavirus gastroenteritis is the leading cause of severe diarrhoea among young children < 5 years old. Previous cost-effectiveness analyses on rotavirus (RV) vaccination in Thailand have generated conflicting results. The aim of this current study is to evaluate the economic impact of introducing RV vaccination in Thailand, using updated Thai epidemiological and cost data.
Methods
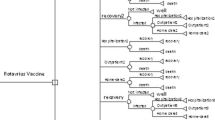
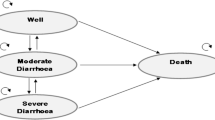
Both cost-utility analysis (CUA) and budget impact analysis (BIA) of human rotavirus vaccine (HRV) under a universal mass vaccination (UMV) programme were conducted. A published static, deterministic, cross-sectional population model was adapted to assess costs and health outcomes associated with RV vaccination among Thai children < 5 years old during 1 year for CUA and over a 5-year period (2019–2023) for BIA. Data identified through literature review were incorporated into the model after consultation with local experts. Base case CUA was conducted from a societal perspective with quality-adjusted life year (QALY) discounted at 3% annually. Scenario analyses as well as one-way and probabilistic sensitivity analyses were conducted to assess the robustness of the base case CUA results. Costs were updated to 2017.
Results
At 99% coverage, HRV vaccination would substantially reduce RV-related disease burden. With an incremental cost-effectiveness ratio (ICER) of Thai baht (THB) 49,923/QALY gained, HRV vaccination versus no vaccination was cost-effective when assessed against a local threshold of THB 160,000/QALY gained. Scenario and sensitivity analyses confirmed the cost-effectiveness with all resultant ICERs falling below the willingness-to-pay threshold. HRV use in the UMV programme was estimated to result in a net expenditure of about THB 255–281 million to the Thai government in the 5th year of the programme, depending on vaccine uptake.
Conclusion
HRV vaccination is estimated to be cost-effective in Thailand. The budget impact following inclusion of HRV into the UMV programme is expected to be partially offset by substantial reductions in RV-related disease costs.
Funding
GlaxoSmithKline Biologicals SA
GSK Study Identifier
HO-17-18213


Similar content being viewed by others
References
Glass RI, Parashar UD, Bresee JS, et al. Rotavirus vaccines: current prospects and future challenges. Lancet. 2006;368(9532):323–32.
Dennehy PH. Transmission of rotavirus and other enteric pathogens in the home. Pediatr Infect Dis J. 2000;19(10):S103–5.
Kawai K, O’Brien MA, Goveia MG, Mast TC, El Khoury AC. Burden of rotavirus gastroenteritis and distribution of rotavirus strains in Asia: a systematic review. Vaccine. 2012;30(7):1244–54.
Kittigul L, Swangsri T, Pombubpa K, Howteerakul N, Diraphat P, Hirunpetcharat C. Rotavirus infection in children and adults with acute gastroenteritis in Thailand. Southeast Asian J Trop Med Public Health. 2014;45(4):816–24.
Jiraphongsa C, Bresee JS, Pongsuwanna Y, et al. Epidemiology and burden of rotavirus diarrhea in thailand: results of sentinel surveillance. J Infect Dis. 2005;192(Supplement_1):S87–93.
World Health Organization (WHO). Rotavirus vaccines WHO position paper—January 2013. Wkly Epidemiol Rec. 2013;5(8):49–64. http://www.who.int/wer/2013/wer8805.pdf?ua=1. Accessed 18 Jul 18.
Ministry of Public Health—Thailand. Product Review (Rotarix). http://pertento.fda.moph.go.th/FDA_SEARCH_DRUG/SEARCH_DRUG/pop-up_drug.aspx?Newcode_U=U1DR1C1012480011711C. Accessed 28 Sep 2018.
Ministry of Public Health—Thailand. Product Review (Rotateq). http://pertento.fda.moph.go.th/FDA_SEARCH_DRUG/SEARCH_DRUG/pop-up_drug.aspx?Newcode_U=U1DR1C10B2510002311C. Accessed 28 Sep 18.
European Medicine Agency (EMA). Rotarix: EPAR—Product information London, UK: European Medicines Agency; 2017. https://www.ema.europa.eu/medicines/human/EPAR/rotarix. Accessed 28 Sep 18.
European Medicine Agency (EMA). Rotateq: EPAR—Product information London, UK: European Medicines Agency; 2017. https://www.ema.europa.eu/medicines/human/EPAR/rotateq. Accessed 28 Sep 18.
Gastanaduy PA, Contreras-Roldan I, Bernart C, et al. Effectiveness of monovalent and pentavalent rotavirus vaccines in guatemala. Clin Infect Dis. 2016;62(Suppl 2):S121–6.
Immergluck LC, Parker TC, Jain S, et al. Sustained effectiveness of monovalent and pentavalent rotavirus vaccines in children. J Pediatr. 2016;172(116–20):e1.
Jonesteller CL, Burnett E, Yen C, Tate JE, Parashar UD. Effectiveness of rotavirus vaccination: a systematic review of the first decade of global post-licensure data, 2006–2016. Clin Infect Dis. 2017;65(5):840–50.
Leshem E, Lopman B, Glass R, et al. Distribution of rotavirus strains and strain-specific effectiveness of the rotavirus vaccine after its introduction: a systematic review and meta-analysis. Lancet Infect Dis. 2014;14(9):847–56.
Payne DC, Selvarangan R, Azimi PH, et al. Long-term consistency in rotavirus vaccine protection: RV5 and RV1 vaccine effectiveness in US children, 2012–2013. Clin Infect Dis. 2015;61(12):1792–9.
Uhlig U, Kostev K, Schuster V, Koletzko S, Uhlig HH. Impact of rotavirus vaccination in Germany: rotavirus surveillance, hospitalization, side effects and comparison of vaccines. Pediatr Infect Dis J. 2014;33(11):e299–304.
World Health Organization (WHO). Detailed review paper on rotavirus vaccines. Geneva, Switzerland: World Health Organization (WHO); 2009. http://www.who.int/immunization/sage/3_Detailed_Review_Paper_on_Rota_Vaccines_17_3_2009.pdf. Accessed 03 Jan 17.
Rosillon D, Buyse H, Friedland LR, Ng S-P, Velázquez FR, Breuer T. Risk of Intussusception After Rotavirus Vaccination: meta-analysis of Postlicensure Studies. Pediatr Infect Dis J. 2015;34(7):763–8.
Calnan M, Krishnarajah G, Duh MS, et al. Rotavirus vaccination in a Medicaid infant population from four US states: compliance, vaccination completion rate, and predictors of compliance. Hum Vaccin Immunother. 2016;12(5):1235–43.
Krishnarajah G, Davis EJ, Fan Y, Standaert BA, Buikema AR. Rotavirus vaccine series completion and adherence to vaccination schedules among infants in managed care in the United States. Vaccine. 2012;30(24):3717–22.
Krishnarajah G, Landsman-Blumberg P, Eynullayeva E. Rotavirus vaccination compliance and completion in a Medicaid infant population. Vaccine. 2015;33(3):479–86.
Luna-Casas G, Juliao P, Carreno-Manjarrez R, et al. Vaccine coverage and compliance in Mexico with the two-dose and three-dose rotavirus vaccines. Hum Vaccin Immunother. 2018. https://doi.org/10.1080/21645515.2018.1540827.
Chotivitayatarakorn P, Chotivitayatarakorn P, Poovorawan Y. Cost-effectiveness of rotavirus vaccination as part of the national immunization program for Thai children. Southeast Asian J Trop Med Public Health. 2010;41(1):114–25.
Muangchana C, Riewpaiboon A, Jiamsiri S, Thamapornpilas P, Warinsatian P. Economic analysis for evidence-based policy-making on a national immunization program: a case of rotavirus vaccine in Thailand. Vaccine. 2012;30(18):2839–47.
Tharmaphornpilas P, Jiamsiri S, Boonchaiya S, et al. Evaluating the first introduction of rotavirus vaccine in Thailand: moving from evidence to policy. Vaccine. 2017;35(5):796–801.
Tharmaphornpilas P, Jiamsiri S, Tuntiwitayapun S, Boonchaiya S. [Effectiveness and Cost-effectiveness of Rotavirus Vaccine in Pilot Provinces (Petchaboon and Sukhothai)] 2015. http://doi.nrct.go.th/ListDoi/Download/299915/570791963bf3929c4c93a81c5c2cce79?Resolve_DOI=10.14455/NRCT.res.2015.121. Accessed 18 Jul 18.
Health Intervention and Technology Assessment Program (HITAP). Guidelines for Health Technology Assessment in Thailand (Second Edition). Bangkok, Thailand: The Medical Association of Thailand; 2014. http://www.hitap.net/wp-content/uploads/2017/06/Thai-HTA-guideline-UPDATES-Jmed-with-Cover.pdf. Accessed 17 Jul 18.
Alkoshi S, Maimaiti N, Dahlui M. Cost-effectiveness analysis of rotavirus vaccination among Libyan children using a simple economic model. Libyan J Med. 2014;9(1):26236.
Bakir M, Standaert B, Turel O, Bilge ZE, Postma M. Estimating and comparing the clinical and economic impact of paediatric rotavirus vaccination in Turkey using a simple versus an advanced model. Vaccine. 2013;31(6):979–86.
Lee I-H, Standaert B, Nievera MC, Rogacion J. Cost-effectiveness analysis of universal mass vaccination with Rotarix® in the Philippines. Ped Inf Dis Soc Phil. 2014;15(1):15–29. https://docs.google.com/viewerng/viewer?url=http://www.pidsphil.org/pdf/Journal_08312014/jo46_ja03.pdf. Accessed 01 Oct 18.
Goossens LM, Standaert B, Hartwig N, Hovels AM, Al MJ. The cost-utility of rotavirus vaccination with Rotarix (RIX4414) in the Netherlands. Vaccine. 2008;26(8):1118–27.
Standaert B, Parez N, Tehard B, Colin X, Detournay B. Cost-effectiveness analysis of vaccination against rotavirus with RIX4414 in France. Appl Health Econ Health Policy. 2008;6(4):199–216.
Chandran A, Fitzwater S, Zhen A, Santosham M. Prevention of rotavirus gastroenteritis in infants and children: rotavirus vaccine safety, efficacy, and potential impact of vaccines. Biologics. 2010;4:213–29.
Ho AM, Nelson EA, Walker DG. Rotavirus vaccination for Hong Kong children: an economic evaluation from the Hong Kong Government perspective. Arch Dis Child. 2008;93(1):52–8.
Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354(1):11–22.
World Health Organization (WHO). Rotavirus vaccines WHO position paper—summary 2013. https://www.who.int/immunization/position_papers/PP_rotavirus_january_2013_summary.pdf. Accessed 01 Apr 19.
Tate JE, Mwenda JM, Parashar UD. Intussusception after rotavirus vaccination in Africa. N Engl J Med. 2018;379(13):1288–9.
Kotirum S, Vutipongsatorn N, Kongpakwattana K, Hutubessy R, Chaiyakunapruk N. Global economic evaluations of rotavirus vaccines: a systematic review. Vaccine. 2017;35(26):3364–86.
Nimdet K, Ngorsuraches S. Willingness to pay per quality-adjusted life year for life-saving treatments in Thailand. BMJ Open. 2015;5(10):e008123.
Leelahavarong P. Budget impact analysis. J Med Assoc Thai. 2014;97:S65–71.
Mohara A, Youngkong S, Velasco RP, et al. Using health technology assessment for informing coverage decisions in Thailand. J Comp Eff Res. 2012;1(2):137–46.
Official Statistics Registration Systems. [Population and housing statistics—Population age] 2018. http://stat.dopa.go.th/stat/statnew/upstat_age.php. Accessed 30 Jan 18.
Loganathan T, Ng CW, Lee WS, Jit M. The hidden health and economic burden of rotavirus gastroenteritis in Malaysia: an estimation using multiple data sources. Pediatr Infect Dis J. 2016;35(6):601–6.
World Health Organization (WHO). Child rotavirus deaths by country 2000–2013 Geneva, Switzerland: World Health Organization (WHO); 2016. 15-Apr-16. http://www.who.int/entity/immunization/monitoring_surveillance/rotavirus_deaths_by_country_2000-2013.xlsx. Accessed 12 Oct 17.
Ministry of Public Health. Public Health Statistics 2016. http://bps.moph.go.th/new_bps/sites/default/files/health_strategy2559.pdf. Accessed 07 Dec 17.
Li RC, Huang T, Li Y, et al. Human rotavirus vaccine (RIX4414) efficacy in the first two years of life: a randomized, placebo-controlled trial in China. Hum Vaccin Immunother. 2014;10(1):11–8.
Burnett E, Parashar U, Tate J. Rotavirus vaccines: effectiveness, safety, and future directions. Paediatr Drugs. 2018;20(3):223–33.
Lopman BA, Pitzer VE. Waxing understanding of waning immunity. J Infect Dis. 2018;217(6):851–3.
Phua KB, Lim FS, Lau YL, et al. Rotavirus vaccine RIX4414 efficacy sustained during the third year of life: a randomized clinical trial in an Asian population. Vaccine. 2012;30(30):4552–7.
The World Bank. Thailand now an upper middle income economy Washington DC, USA The World Bank Group; 2011. http://www.worldbank.org/en/news/press-release/2011/08/02/thailand-now-upper-middle-income-economy. Accessed 23 May 18.
UN Inter-agency Group for Child Mortality Estimation. Child Mortality Estimates 2016. http://www.childmortality.org/. Accessed 02 Oct 18.
World Health Organization (WHO). Global Health Observatory Data, Under-five mortality Geneva, Switzerland. 2018. http://www.who.int/gho/child_health/mortality/mortality_under_five_text/en/. Accessed 23 May 18.
World Health Organization (WHO). Expanded Program on Immunization (EPI) Fact Sheet 2017: 16. http://www.searo.who.int/immunization/data/thailand_2017.pdf. Accessed 30 Jan 18.
Riera-Montes M, O’Ryan M, Verstraeten T. Norovirus and rotavirus disease severity in children: systematic review and meta-analysis. Pediatr Infect Dis J. 2017;37(6):501–5.
Ministry of Commerce—Thailand. Report for Consumer Price Index of Thailand 2009. http://www.indexpr.moc.go.th/price_present/TableIndexG_region.asp?table_name=cpig_index_country&province_code=5&type_code=g&check_f=i&year_base=2558&nyear=2552. Accessed 30 Jan 18.
Ministry of Commerce—Thailand. Report for Consumer Price Index of Thailand 2014. http://www.indexpr.moc.go.th/price_present/TableIndexG_region.asp?table_name=cpig_index_country&province_code=5&type_code=g&check_f=i&year_base=2558&nyear=2557. Accessed 30 Jan 18.
Ministry of Commerce - Thailand. Report for Consumer Price Index of Thailand 2017. http://www.indexpr.moc.go.th/price_present/TableIndexG_region.asp?table_name=cpig_index_country&province_code=5&type_code=g&check_f=i&year_base=2558&nyear=2560. Accessed 30 Jan 18.
Riewpaiboon A. Measurement of costs for health economic evaluation. J Med Assoc Thai. 2014;97:S17–26.
Bank of Thailand. Rates of Exchange of Commercial Banks in Bangkok Metropolis (2002-present). 2017. http://www2.bot.or.th/statistics/ReportPage.aspx?reportID=123&language=eng. Accessed 21 Feb 18.
Riewpaiboon A. Standard cost lists for health economic evaluation in Thailand. J Med Assoc Thai. 2014;97(Suppl 5):S127–34.
Saokaew S, Permsuwan U, Chaiyakunapruk N, Nathisuwan S, Sukonthasarn A, Jeanpeerapong N. Cost-effectiveness of pharmacist-participated warfarin therapy management in Thailand. Thromb Res. 2013;132(4):437–43.
World Health Organization (WHO). Global Health Estimates 2000–2015 Geneva, Switzerland: World Health Organization (WHO). http://www.who.int/healthinfo/global_burden_disease/en/. Accessed 24 Jul 18.
World Health Organization (WHO). WHO Methods for Life Expectancy and Healthy Life Expectancy. Geneva, Switzerland: World Health Organization (WHO); 2014. http://www.who.int/healthinfo/statistics/LT_method_1990_2012.pdf. Accessed 24 Jul 18.
World Health Organization (WHO). Global Health Observatory Data Repository Geneva, Switzerland: World Health Organization (WHO); 2018. http://apps.who.int/gho/data/view.main.61640?lang=en. Accessed 24 Jul 17.
Rochanathimoke O, Riewpaiboon A, Postma MJ, Thinyounyong W, Thavorncharoensap M. Health related quality of life impact from rotavirus diarrhea on children and their family caregivers in Thailand. Expert Rev Pharmacoecon Outcomes Res. 2018;18(2):215–22.
Chaikledkaew U. Presentation of economic evaluation results. J Med Assoc Thai. 2014;97(Suppl 5):S72–80.
Permsuwan U, Guntawongwan K, Buddhawongsa P. Handling time in economic evaluation studies. J Med Assoc Thai. 2008;91(Suppl 2):S53–8.
Limwattananon S. Sensitivity analysis for handling uncertainty in an economic evaluation. J Med Assoc Thai. 2014;97(Suppl 5):S59–64.
Acknowledgements
Trademark
Rotarix is a trademark of the GSK group of companies. Rotateq is a trademark of Merck & Co. Inc.
Funding
GlaxoSmithKline Biologicals SA was the funding source and was involved in all stages of the study conduct and analysis (GSK study identifier: HO-17-18213). GlaxoSmithKline Biologicals SA also took over all costs associated with the development and publication of the present manuscript. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Medical writing, editorial, and other assistance
The authors thank Prof. Somsak Lolekha (Mahidol University, Thailand) and Claire Montante (GSK, Belgium) for their contribution to the study as well as Rebecca Crawford (GSK, Singapore) for her support during the publication development. The authors also thank Business & Decision Life Sciences platform for editorial assistance and manuscript coordination on behalf of GSK: Nathalie Arts coordinated manuscript development and editorial support and Sarah Fico provided medical writing support.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Wasana Prasitsuebsai is an employee of and holds shares in the GSK group of companies. Gyneth Lourdes Bibera is an employee of and holds shares in the GSK group of companies. Xu-Hao Zhang is an employee of and holds shares in the GSK group of companies. Kyu-Bin Oh is an employee of and holds shares in the GSK group of companies. Christa Lee is an employee of the GSK group of companies. Surasak Saokaew and Kirati Kengkla have nothing to disclose.
Compliance with Ethics Guidelines
This article does not contain any studies with human participants or animals performed by any of the authors.
Data Availability
GSK makes available anonymized individual participant data and associated documents from interventional clinical studies which evaluate medicines, upon approval of proposals submitted to http://www.clinicalstudydatarequest.com. To access data for other types of GSK sponsored research, for study documents without patient-level data and for clinical studies not listed, please submit an enquiry via the website.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.7998818.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Saokaew, S., Prasitsuebsai, W., Bibera, G.L. et al. Economic Evaluation of Human Rotavirus Vaccine in Thailand. Infect Dis Ther 8, 397–415 (2019). https://doi.org/10.1007/s40121-019-0246-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-019-0246-1