Abstract
Introduction
Pneumococcal conjugate vaccines (PCVs) have been available in Canada since 2001, with 13-valent PCV (PCV13) added to the infant routine immunization program throughout all Canadian provinces by 2011. The use of PCVs has dramatically reduced the burden of pneumococcal disease in Canada. As a result, decision-makers may consider switching from a more costly, higher-valent vaccine to a lower-cost, lower-valent vaccine in an attempt to allocate funds for other vaccine programs. We assessed the health and economic impact of switching the infant vaccination program from PCV13 to 10-valent PCV (PCV10) in the context of the Canadian health care system.
Methods
We performed a review of Canadian databases supplemented with published and unpublished data to obtain the historical incidence of pneumococcal disease and direct and indirect medical costs. Observed invasive pneumococcal disease (IPD) trends from surveillance data were used as a basis to forecast the future number of cases of IPD, pneumococcal pneumonia, and acute otitis media given a PCV13- or PCV10-based program. Costs and outcomes over 10 years were then estimated and presented in 2017 Canadian dollars discounted at 3% per year.
Results
Switching from PCV13 to PCV10 would result in an additional 762,531 cases of pneumococcal disease over 10 years. Although PCV13 has a higher acquisition cost, switching to PCV10 would increase overall costs by over $500 million. Forecasted overall disease incidence was estimated substantially higher with PCV10 than with PCV13 primarily because of the potential reemergence of serotypes 3 and 19A. PCV13 was also cost saving compared with PCV10, even within a 5-year time horizon. Probabilistic sensitivity analysis showed that a PCV13-based program remained cost saving in all simulations.
Conclusion
Although switching to a PCV10-based infant vaccination program in Canada might result in lower acquisition costs, it would also result in higher public health cost and burden because of serotype reemergence.
Funding
Pfizer Inc.
Similar content being viewed by others
Introduction
Streptococcus pneumoniae is a gram-positive bacterium with more than 90 serotypes associated with diseases such as acute otitis media (AOM), pneumonia, and invasive pneumococcal disease (IPD), such as bacteremia and meningitis [1]. Although IPD is more severe and has a greater chance of leading to mortality, pneumonia and AOM represent a significant portion of the burden of disease and associated medical costs [2, 3].
Since the early 2000s, pneumococcal conjugate vaccines (PCVs) containing 7 (PCV7, Prevnar/®, Wyeth Lederle Vaccines), 10 (PCV10, Synflorix®, GlaxoSmithKline Biologicals S.A.), and 13 serotypes (PCV13, Prevnar 13®, Wyeth/Pfizer Vaccines) have been developed and implemented worldwide for use in routine infant immunization programs. Details on the serotypes covered by each vaccine can be seen in the online Supporting Information (S1 Table 7). The use of these vaccines has substantially reduced the burden of vaccine-type pneumococcal disease. In Canada, PCV7 received regulatory approval from Health Canada in June 2001. The National Advisory Committee on Immunization (NACI) makes recommendations for the use of vaccines. Individual provinces are responsible for implementation and funding of the NACI recommendation, and by 2005, PCV7 was included in routine infant immunization schedules across all provinces. Cases of IPD due to the PCV7 serotypes dramatically decreased following the introduction of PCV7 [4, 5]. However, non-PCV7 serotypes began to emerge, with a statistically significant increase for serotype 19A [4]. PCV10, which does not cover serotype 19A, replaced PCV7 as the vaccine of choice in 2009 in many provinces for a brief period of time. However, in 2010, NACI reviewed the epidemiology of IPD in Canada and recommended PCV13 as the product of choice for routine infant immunization to address the increased burden of illness due to serotypes contained in PCV13 but not in PCV7 and PCV10, particularly 19A [6]. By 2011, PCV13 was included in the routine infant immunization schedules of all provinces.
As it covers a larger number of serotypes, PCV13 may provide greater value with respect to disease prevention but at a higher acquisition cost than PCV10. As government-funded programs are periodically reevaluated within the context of scarce health care resources, switching to a lower-valent, less-costly vaccine could be an option to help contain costs and in the context of decreasing burden of pneumococcal disease due to the success of vaccination. However, switching from PCV13 to PCV10, a vaccine that does not contain serotypes 3, 6A, and 19A, may have undesirable consequences, such as resurgence of these serotypes (that are still circulating within the Canadian population). Specifically, 19A is known for its link with complicated disease, multidrug resistance and the need for longer antimicrobial treatment [7].
Although previous cost-effectiveness analyses in Canada and other countries have found PCV13 to be cost saving compared with PCV10 [8,9,10,11,12,13], most of these models have been developed to evaluate the impact of introducing a PCV immunization program, and none have evaluated the impact of switching between two vaccination programs. Understanding the potential impact of a program switch will give decision-makers relevant additional information about the program’s clinical and economic value. The objective of this study was to assess the impact of switching the infant pneumococcal vaccination program from the use of PCV13 to PCV10 in the context of the Canadian health care system.
Methods
We developed a decision-analytic model in Microsoft Excel using secondary data analysis. This article does not involve the study of human participants or animals performed by any of the authors and as such was not subject to institutional review board approval. Table 1 summarizes all inputs used in the model.
Population and Comparators
The model compares the impact of implementing an infant vaccination program in which the pneumococcal vaccine of choice is switched to PCV10 versus continued use of PCV13. The population of Canada (35,848,610) is included in the analysis, in which 776,370 children < 2 years of age are eligible for pneumococcal vaccination (Table 1). Eighty-five percent of infants were assumed to be vaccinated each year [14]. The population was stratified into seven age groups: < 2, 2–4, 5–17, 18–34, 35–49, 50–64, and 65+ years.
Model Structure
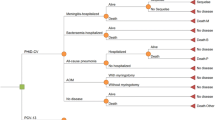
A decision-analytic model (Fig. 1) was developed to estimate the public health and economic impact of potential changes in incidence of pneumococcal disease should Canada switch from a PCV13-based infant vaccination program to a PCV10-based program. Specifically, the model used historical IPD surveillance data by age group and serotype to project the serotype and disease incidence given vaccination with PCV10 or PCV13 so that the costs and clinical impact of each vaccination program can be calculated. In addition, we estimated cases of pneumococcal AOM and pneumococcal pneumonia as a function of IPD incidence.
Estimating IPD Cases
For forecasting the number of IPD cases, historical, age- and serotype-specific surveillance data were obtained. Nationwide population-based surveillance data are not available in Canada. As such, age- and serotype-specific IPD incidences were obtained from the Toronto Invasive Bacterial Disease Network population-based active surveillance data set and extrapolated to the Canadian population. IPD surveillance data were collected from 2001 to 2015, a time period spanning the use of PCV7, PCV10, and PCV13. “Covered periods” represent periods in which a vaccine that covered a specific serotype was in use within the vaccination program, and “noncovered periods” represent periods in which no vaccine or a vaccine that did not cover a specific serotype was used in the vaccination program. The direct and indirect effects of a specific vaccine were assumed to be implicitly captured within these surveillance data.
For forecasting cases of invasive disease, serotype- and age-group-specific trend lines were independently fit to historical data of covered and noncovered periods. For example, for serotypes 3, 6A, and 19A, noncovered trend lines were based on data prior to 2011 (i.e., the years prior to PCV13 introduction). The trend line equations were created based on the best data fit (highest R-squared) from a set of standard distributions. Trend lines for covered periods were based on linear, logarithmic, exponential, and power functions. Trend lines for noncovered periods were conservatively based on linear and logarithmic functions to reduce the risk of projecting immediate, unrealistically large increases in disease incidence.
Additional assumptions were made about the forecasting. When the vaccination program removed vaccine pressure on a serotype (e.g., when PCV13 is switched to PCV10, serotype 19A becomes noncovered), it is possible that the indirect effects (from individuals previously vaccinated with PCV13) and low prevalence of pneumococcal carriage would potentially delay the resurgence of disease. To address this possibility, a 2-year lag (Fig. 1) was assumed before serotype reemergence would begin after a switch to PCV10.
Additionally, an upper limit on forecasted incidence was applied, as it was assumed that the forecasted disease reemergence in each age group would not exceed the highest incidence reported in the non-covered periods of the historical data (Fig. 1). This upper limit was included because whether the IPD incidence would increase beyond pre-PCV levels given a new serotype distribution is not known. The projected IPD trend lines for each age group and serotype are presented in the online Supporting Information (S1 Tables 2–7) for the covered and noncovered periods. Historical IPD surveillance data included all invasive disease. The model considered IPD a combination of meningitis and bacteremia based on the respective age-specific proportions of each disease to estimate the associated costs and outcomes of each health state [16] (Table 1).
Estimating Pneumonia Cases
Incidence of all-cause hospitalized and nonhospitalized pneumonia was obtained from the Canadian Institute for Health Information (CIHI) Discharge Abstract database (OXON, 2011 and 2014) (Table 2). Historical incidence of all-cause pneumonia was available from 2001 to 2014.
Because data are not available on serotype-specific pneumococcal pneumonia, it was assumed that 20% of all-cause hospitalized and 20% of all-cause nonhospitalized pneumonia were due to S. pneumoniae with ratios constant over time. This was estimated based on the range used in Morrow, De Wals [18] (13–37%), assuming several years of PCV13 use and therefore a reduction in the burden of pneumococcal pneumonia. The numbers of hospitalized pneumococcal pneumonia and nonhospitalized pneumococcal pneumonia cases each year were estimated based on the same relative change in IPD cases forecasted each year. The direct and indirect effects of vaccination across all age groups are implicitly considered through the IPD data and were correspondingly considered in the base-case analysis for hospitalized pneumonia. We assumed no effects of vaccination on incidence of nonhospitalized pneumonia.
Estimating AOM Cases
Due to limited availability of data specific to pneumococcal AOM incidence, the approach for estimating pneumococcal AOM incidence was similar to the approach for pneumonia. The incidence of all-cause AOM was obtained from published literature (Table 2) [18, 26] but was assumed to only occur in individuals < 5 years of age. The proportion of all-cause AOM incidence that is pneumococcal was conservatively assumed to be a constant 20% to be consistent with estimates for pneumonia [18]. AOM incidence was assumed to vary proportionately with IPD. The number of pneumococcal AOM cases was obtained by multiplying the proportion of pneumococcal AOM cases by the relative change in IPD cases forecasted each year. This methodology implicitly accounts for both direct and indirect effects of vaccination for those < 5 years of age. Though data exist suggesting that PCVs may have an impact against AOM caused by nonpneumococcal pathogens such as nontypeable Haemophilus influenza (NTHi) [27], this analysis assumed that the impact was limited to serotypes contained in the vaccines.
Mortality
General population all-cause mortality was derived using the demographics and number of deaths reported by Statistics Canada for 2015/2016 [15, 19]. Additional mortality due to IPD and hospitalized pneumonia was obtained from Jetté, Delage [20] and Scheifele, Halperin [21] (Table 1). Occurrence of AOM and nonhospitalized pneumonia was assumed not to increase mortality.
Disease Sequelae
Pneumococcal disease may result in clinical sequelae such as neurologic impairment and hearing loss. For meningitis, hearing loss and neurologic impairment were included for 13 and 7% of patients, respectively [28, 29]. Sequelae of bacteremia or pneumonia were not considered. For AOM, 5% of patients were assumed to require myringotomy procedures [18].
Costs
Costs included vaccine acquisition and administration, direct and indirect disease-related costs, and myringotomy procedure. Potential additional costs of complications associated with meningitis were not included. In Canada, publicly funded vaccines are exclusively contracted with group purchasing organizations under confidential terms. The price of PCV10 was estimated to be 30% lower than PCV13 [30, 31]. Cost of administration was estimated to be $6.63 [22, 32]. Direct and indirect costs for each disease by age group [17, 18] are summarized in Table 1. Indirect costs were estimated as lost productivity due to cases of disease for patients, parents, and/or caregivers affected by the disease using the human capital approach [17, 23, 33]. All costs were adjusted to 2017 values and discounted at a rate of 3% [22, 34].
Utility Inputs
We assumed an age-specific baseline utility weight for individuals in each age group who did not experience a case of disease. Utilities were estimated from the general Canadian population and used in a previous Canadian-specific cost-effectiveness analysis [9] (Table 1).
Utility decrements were applied for each occurrence of disease. Annual decrements of 0.0079 and 0.0232 were assumed for bacteremia and meningitis, respectively [35]. Decrements of 0.0050, 0.0040, and 0.0060 were assumed for AOM, nonhospitalized pneumonia, and hospitalized pneumonia, respectively [16]. Meningitis-related sequelae utility decrements were considered for neurologic impairment (0.40) and hearing loss (0.20) [18, 36]. Utilities were also discounted at a rate of 3% [34].
Base-Case Calculations
The projected number of cases, life-years, quality-adjusted life-years (QALY), and costs incurred with continuing the PCV13-based vaccination program was compared with the number of outcomes and costs that might be incurred if Canada switched to a PCV10-based vaccination program. Incremental cost per life-year gained and incremental cost per QALY gained were calculated.
Sensitivity Analyses
To test the robustness of the model assumptions and specific parameters used in the analysis, we examined the effect of changing parameters and assumptions in one-way sensitivity analyses. Individual parameters and assumptions varied based on 95% confidence intervals (CIs), plausible ranges from the literature, or ± 20% when neither CIs nor plausible ranges were available. Results were plotted on tornado diagrams in which inputs were presented from most to least sensitive.
Series of scenario and multi-way sensitivity analyses were also performed to examine the impact of varying combinations of parameters and assumptions (Supporting Information). Specifically, due to the uncertainty surrounding serotype replacement and to avoid under- or overestimation of vaccine impact, a number of scenarios were tested by varying trend lines based on historical surveillance data from the UK and USA to reflect PCV13 infant vaccination and The Netherlands and Finland to reflect PCV10 infant vaccination [37,38,39,40,41,42,43]. These countries were chosen because PCVs have been used with high uptake over a long period of time, and each of these countries has a robust surveillance system in place. Additionally, we considered a time horizon of 5 years, a scenario excluding indirect costs, and a scenario in which indirect effects for hospitalized pneumonia were not considered (i.e., a change in hospitalized pneumonia incidence was constrained to those < 5 years of age). Finally, to consider the possibility of having reached an equilibrium with PCV13 use, we considered a scenario in which disease incidence under PCV13 use had reached a minimum in all age groups and thus any changes in incidence with PCV13 use would be increases as a result of serotype replacement.
In addition to one- and multi-way sensitivity analyses, probabilistic sensitivity analyses were performed (second-order Monte Carlo simulations). The proportion of IPD that is meningitis, vaccination rate, percentage of all-cause AOM that is pneumococcal AOM, percentage of all-cause pneumonia that is pneumococcal pneumonia, utilities, and disease-specific mortality were drawn from a beta distribution. Limits on forecasted incidence, time to disease reemergence, direct costs, vaccine acquisition costs, vaccine administration costs, myringotomy procedure cost, lost productivity, and number of general deaths were drawn from a gamma distribution. Analyses were run 10,000 times to ensure stability in the results for each relevant scenario.
Results
Base-Case Results
If Canada switches from a PCV13- to PCV10-based infant vaccination program, 762,531 more cases of disease are predicted to occur because of serotype reemergence (Table 3) over the next 10 years. The largest number of cases prevented with continued use of PCV13 was observed for AOM (709,073 cases prevented) followed by hospitalized pneumococcal pneumonia (38,556), nonhospitalized pneumococcal pneumonia (10,285), and bacteremia (3009). The increase in cases with PCV10 resulted in reduced life-years (− 12,021) and QALYs (− 10,948) compared with continued use of PCV13.
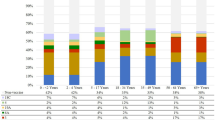
For both vaccination strategies, most of the forecasted cases of IPD occurred in individuals < 2 and ≥ 65 years of age (Fig. 2a, b; for other age groups, see online Supporting Information S1 Fig. 1a–c). Noncovered serotypes represented the majority of disease incidence in both of these age groups. In those < 2 years of age, overall disease incidence was estimated to increase substantially more with a PCV10-based program than with a PCV13-based program.
a Invasive pneumococcal disease incidence over time for persons < 2 years of age. b Invasive pneumococcal disease incidence over time for persons ≥ 65 years of age. IPD Invasive pneumococcal disease, PCV7 7-valent pneumococcal conjugate vaccine, PCV10 10-valent pneumococcal conjugate vaccine, PCV13 13-valent pneumococcal conjugate vaccine
Despite a 30% higher cost of vaccination with PCV13 (increase of $180 million over 10 years), the costs of treating cases of disease more than offset these costs. Specifically, maintaining vaccination with PCV13 was estimated to save at least $500 million over a 10-year period.
Sensitivity Analyses
The one-way sensitivity analysis (Fig. 3) demonstrated that a PCV13-based program remained cost saving over a 10-year horizon as parameters changed within their plausible range. The model results varied most with changes in the baseline utility weight and pneumonia case-fatality rate for adults ≥ 65 years of age, the percentage of AOM caused by S. pneumoniae in children 2–4 years of age, and the direct medical costs of pneumonia. However, a PCV13-based program remained cost saving across all individual parameter variation.
In scenario analyses, we observed that even over a 5-year time horizon or when excluding indirect effects in hospitalized pneumonia, a PCV13-based vaccination program remained more cost-saving than a PCV10-based vaccination program. When data from the UK and US were used to forecast cases of disease for PCV13 and The Netherlands and Finland data were used to forecast cases of disease for PCV10, a PCV13-based program remained cost saving (Table 4; differences in trend lines over time can be seen in S1 Fig. 2 and S1 Fig. 3) in all but the scenario when UK data were used for PCV13 and The Netherlands data were used for PCV10. Using data from the US to forecast PCV13 trends resulted in greater cost savings and health gains with continued use of PCV13 versus a switch to PCV10. Finally, in a scenario excluding indirect costs (not shown), PCV13 remained a cost-saving strategy and was estimated to save the Canadian health system approximately $350 million over a 10-year period.
The probabilistic sensitivity analysis showed that a PCV13-based program remained dominant in 100% of the simulations (Fig. 4). Mean difference in costs and QALYs were − $523,884,782 (95% CI − $414,683,656 to − $649,241,715) and 11,037 (95% CI 8468–14,125), respectively.
Discussion
We developed a decision-analytic model to forecast and compare the public health and economic impact of sustained use of PCV13 with a change in PCV recommendation to a lower-valent vaccine (i.e., PCV10) in the infant vaccination program in Canada. The analysis estimated that continuing the PCV13 program would be cost saving within both the 5- and 10-year time horizons and would provide better outcomes compared with switching to PCV10. The expected increase in cases of disease with a switch to PCV10 was predominantly a result of increases in incidence of cases because of serotypes 3 and 19A in the < 2- and ≥ 65-year-old populations. PCV13 remained the most cost-saving strategy in nearly all scenarios when varying parameters and model assumptions within plausible ranges as well as when varying assumptions on vaccine impact and serotype replacement using trend lines from other countries. While we did not consider two-way sensitivity analyses, the results of the one-way analysis, in which PCV13 remained dominant in all cases, suggest that a two-way sensitivity analysis would not change the cost-effectiveness of PCV13. A three-way sensitivity analysis involving the three most sensitive parameters from the one-way sensitivity analysis still resulted in cost savings with PCV13.
These results are consistent with some previous studies’ findings. Clinical evidence has shown that reducing PCV pressure results in an increase in disease incidence within a very short time period [44, 45]. Waye and Chuck [46] developed an economic model using serotype-specific IPD surveillance data from the Alberta Public Health Laboratory and found that the introduction of PCV13 reduced the number of IPD cases significantly and resulted in cost savings. Two Markov-based cost-effectiveness analyses for a PCV13-based program in Canada showed PCV13 to be dominant over a lifetime horizon compared with a PCV10-based program [8, 47]. However, these models all assumed the same price for PCV10 as PCV13. The results of our study strongly suggest there is economic value in continuing a PCV13-based program, as PCV13 was found to be cost saving despite an assumed 30% price difference between the vaccines. Costs related to long-term sequelae due to bacteremia and meningitis were also omitted. As such, medical cost savings are likely underestimated.
This study adds to the body of evidence surrounding the impact of PCVs and is the first analysis to estimate the impact of changing vaccination programs from a 13- to 10-valent vaccine. Existing analyses utilized prevaccine incidence in a naive population to estimate the impact of introducing a PCV program [8, 11, 47, 48]. However, given significant experience with PCVs in most countries, these methods are no longer appropriate. Previous analyses have also generally used a cohort- or population-based approach that does not fully consider the indirect effects of vaccination and impact of serotype replacement. By modeling each serotype individually by age group at a population level, this analysis more accurately estimates potential serotype replacement in the event of a vaccine switch. This methodology also provides a more realistic picture of how the vaccination programs may behave as it relies on real-world effectiveness data as opposed to the efficacy data used in most existing analyses [8, 11, 47,48,49,50,51]. This means that the forecasts inherently capture vaccine dynamics observed in the real world, such as vaccine waning, cross-reactivity, and herd protection.
Out results are consistent with a comparable analysis recently published by Zhou et al. [52] in which they estimated the future impact of IPD in adults over 65 years of age in Quebec. However, Zhou et al. [52] did not evaluate the costs, impact across all ages, or effect of changing vaccine programs.
As with any modeling exercise, the approach is subject to limitations. A key assumption is that historical serotype trends will determine future disease incidence. In other words, the model assumes disease dynamics will continue to behave similarly to what was observed in the past. This assumption has the benefit of being a conceptually simple and intuitive approach. However, it does not account for natural fluctuations that can be observed in the absence of vaccine pressure, serotype replacement, changes in vaccine uptake, antibiotic prescription use, changes in clinical practice, genetic mutations, or other unanticipated factors. We attempted to mitigate some of these limitations by putting an upper limit on disease reemergence such that it did not exceed the incidence reported in the noncovered periods of the historical data. We also allowed for a delay in reemergence of noncovered serotypes to account for low prevalence of pneumococcal carriage at the time of a switch to PCV10. Additionally, we considered forecasts based on historical surveillance data from other countries (the UK, the US, Finland, and The Netherlands) to explore the sensitivity of the model’s results to different rates of serotype replacement under different dosing schedules and historical experience with PCVs [37, 39, 42, 44]. Given the experience of PCVs has varied across countries, these scenarios account for differences in the effectiveness of each vaccine against vaccine serotypes, potential cross-reactivity (6A and 19A), and non-vaccine type replacement. Consistently, we have seen decreases in disease caused by serotype 19A in countries using PCV13 and increases in countries using PCV10 in both vaccinated and unvaccinated age groups [53]. Recent evidence further demonstrates limited evidence of PCV10 providing cross-protection with serotype 19F [54, 55]. For serotype 3, while indirect effects have been more varied in older age groups with PCV13, results in this study were not significantly impacted by assumptions on serotype 3, and scenario analyses were robust to different forecasting assumptions [42, 56]. For example, despite recent increases in serotype 3 and other non-vaccine serotypes in the UK, results remained consistent using UK trend lines [42].
Another limitation is that, although PCV7 was available in Canada as early as 2001, the implementation of PCV7 as part of the routine infant vaccination program in the provinces occurred at different times. We chose 2005 (the year of implementation in Ontario) as the anchor for the rest of Canada. It is highly probable that some children in Ontario received PCV7 before it was added to the routine immunization program (Pfizer internal data), which would explain why the incidence of disease was already decreasing in 2005 (Fig. 2a, b). However, this does not impact the forecasted trend lines and results because PCV7 serotype disease has largely been eradicated and those serotypes are common between both vaccines.
The analysis assumes that a constant proportion of all-cause pneumonia and AOM disease is caused by S. pneumoniae. Previous analyses assumed a constant incidence of AOM and pneumonia and adjusted all-cause clinical efficacy of PCV7 by the ratio of serotype coverage of the vaccine [11, 57, 58]. As the prevalence of nasopharyngeal carriage declines in the population under vaccine pressure, the shift in the ecologic niche may influence the proportion of pneumonia and AOM caused by S. pneumoniae. Thus, the remaining serotypes observed causing IPD may have a different propensity to cause AOM or pneumonia. Limited data are available to inform such assumptions, and therefore results may be over- or underestimated. However, a similar approach has been used in previous studies to estimate the relationship between invasive and noninvasive pneumococcal disease [59], and results in this study were robust to extensive sensitivity analyses.
The analysis does not consider potential benefits of vaccines on other non-S. pneumoniae bacteria such as NTHi. An investigational 11-valent vaccine in which all serotypes were conjugated to protein D, a highly conserved protein from NTHi, showed statistically significant efficacy against all AOM episodes due to NTHi [60, 61]. Previous economic analyses have assumed an additional benefit for PCV10 in preventing NTHi AOM based on evidence for this investigational 11-valent vaccine [47, 50, 51, 62, 63]. However, real-world effectiveness data published over the last few years have presented mixed evidence. To our knowledge, to date, no study of PCV10 has shown a similar effect as that seen for the investigational 11-valent vaccine in reducing NTHi-caused AOM [64].
Additionally, the analysis did not specifically consider a potential effect of the publicly funded, 23-valent pneumococcal polysaccharide vaccine (PPV23) program for adults ≥ 65 years in Canada. However, any use and impact of PPV23 is inherently captured within the historical IPD surveillance and hospitalized pneumonia data. In addition, the use of PPV23 in adults ≥ 65 years would not change given a change in vaccine in an infant vaccination program. Furthermore, PPV23 was used throughout the history of the PCV infant vaccine program in Canada. Thus, results are likely unaffected, even though the incidence of disease due to serotypes specific to PPV23 has been consistently increasing among adults ≥ 65 years since 2008 [65, 66].
As in all pneumococcal vaccine economic analyses, this study is also subject to the limitation of available data. Specifically, we do not have serotype-specific information for all of Canada. To estimate the serotype-specific incidence for the country as a whole, we assumed that the distribution of cases as seen in the Toronto Invasive Bacterial Disease Network surveillance data is representative of the general Canadian population. The assumption this has on the model results is unclear. However, when performing this analysis using Quebec-specific surveillance data, results were even more favorable for PCV13 [30]. Finally, some newer evidence exists on the disutility associated with pneumonia in adults, but for modeling simplicity this study assumed consistent utility rates for all age groups [67, 68]. However, using these estimates would have only improved results for PCV13, so our estimates should be considered conservative.
Conclusion
This analysis incorporates observed trends in serotype dynamics in the presence and absence of vaccination pressure. The approach is novel and intuitive and relies on observed historical data that may better reflect real-world effectiveness of pneumococcal vaccines given their extended use. Furthermore, the historical incidence data used to drive the analysis inherently considers factors such as herd protection, waning efficacy, and cross-reactivity. This allows for the modeling approach of a very complicated disease to be greatly simplified and more easily understood. The use of a forecasting approach also allows us to easily understand both historical and projected data to observe changes in incidence over time. The results of this analysis estimated that switching from a PCV13- to a PCV10-based infant vaccination program would increase the total incidence of disease due to the reemergence of serotypes 3, 6A, and 19A, the latter known for its link with severe disease and high likelihood of presenting antibiotic resistance. Therefore, switching may lead to a substantial burden on public health and costs to the health care system.
References
Public Health Agency of Canada. Streptococcus pneumoniae. Pathogen safety data sheet—infectious substances. 2011. https://www.canada.ca/en/public-health/services/laboratory-biosafety-biosecurity/pathogen-safety-data-sheets-risk-assessment/streptococcus-pneumoniae.html. Accessed 6 Oct 2017.
Huang SS, Johnson KM, Ray GT, Wroe P, Lieu TA, Moore MR, et al. Healthcare utilization and cost of pneumococcal disease in the United States. Vaccine. 2011;29(18):3398–412.
Petit G, De Wals P, Law B, Tam T, Erickson LJ, Guay M, et al. Epidemiological and economic burden of pneumococcal diseases in Canadian children. Can J Infectious Dis. 2003;14(4):215–20.
Bettinger JA, Scheifele DW, Kellner JD, Halperin SA, Vaudry W, Law B, et al. The effect of routine vaccination on invasive pneumococcal infections in Canadian children, Immunization Monitoring Program, Active 2000–2007. Vaccine. 2010;28(9):2130–6.
Rudnick W, Liu Z, Shigayeva A, Low DE, Green K, Plevneshi A, et al. Pneumococcal vaccination programs and the burden of invasive pneumococcal disease in Ontario, Canada, 1995–2011. Vaccine. 2013;31(49):5863–71.
Public Health Agency of Canada. Canada communicable disease report: an advisory committee statement (ACS) National Advisory Committee on Immunization (NACI) update on the use of conjugate pneumococcal vaccines in childhood. 2010. http://www.phac-aspc.gc.ca/publicat/ccdr-rmtc/10vol36/acs-12/acs-12-eng.pdf. Accessed 6 Oct 2017.
Song JH. Advances in pneumococcal antibiotic resistance. Exp Rev Respir Med. 2013;7(5):491–8.
Earnshaw SR, McDade CL, Zanotti G, Farkouh RA, Strutton D. Cost-effectiveness of 2 + 1 dosing of 13-valent and 10-valent pneumococcal conjugate vaccines in Canada. BMC Infect Dis. 2012;12:101.
Chuck AW, Jacobs P, Tyrrell G, Kellner JD. Pharmacoeconomic evaluation of 10- and 13-valent pneumococcal conjugate vaccines. Vaccine. 2010;28(33):5485–90.
Ordonez JE, Orozco JJ. Cost-effectiveness analysis of the available pneumococcal conjugated vaccines for children under 5 years in Colombia. Cost Effect Res Alloc. 2015;13:6.
Strutton DR, Farkouh RA, Earnshaw SR, Hwang S, Theidel U, Kontodimas S, et al. Cost-effectiveness of 13-valent pneumococcal conjugate vaccine: germany, Greece, and The Netherlands. J Infect. 2012;64(1):54–67.
Newall AT, Creighton P, Philp DJ, Wood JG, MacIntyre CR. The potential cost-effectiveness of infant pneumococcal vaccines in Australia. Vaccine. 2011;29(45):8077–85.
Mucino-Ortega E, Mould-Quevedo JF, Farkouh R, Strutton D. Economic evaluation of an infant immunization program in Mexico, based on 13-valent pneumococcal conjugated vaccines. Value Health J Int Soc Pharmacoecon Outcomes Res. 2011;14(5 Suppl 1):S65–70.
Pfizer. Percentage of infants vaccinated. Data on file. 2017.
Statistics Canada. Table 051–0001—estimates of population, by age group and sex for July 1, Canada, provinces and territories, annual (persons unless otherwise noted). 2016. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1710000501. Accessed 6 Oct 2017.
Melegaro A, Edmunds WJ. Cost-effectiveness analysis of pneumococcal conjugate vaccination in England and Wales. Vaccine. 2004;22(31–32):4203–14.
Ontario Health Data Branch Web Portal. Ontario case costing initiative. 2016. https://hsim.health.gov.on.ca/hdbportal/. Accessed 6 Oct 2017.
Morrow A, De Wals P, Petit G, Guay M, Erickson LJ. The burden of pneumococcal disease in the Canadian population before routine use of the seven-valent pneumococcal conjugate vaccine. Can J Infect Dis Med Microbiol. 2007;18(2):121–7.
Statistics Canada. Table 051–0002—estimates of deaths, by sex and age group, Canada, provinces and territories, annual (persons). 2016. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1710000601. Accessed 6 Oct 2017.
Jetté LP, Delage G, Ringuette L, Allard R, De Wals P, Lamothe F, et al. Surveillance of invasive Streptococcus pneumoniae infection in the province of Quebec, Canada, from 1996 to 1998: serotype distribution, antimicrobial susceptibility, and clinical characteristics. J Clin Microbiol. 2001;39(2):733–7.
Scheifele D, Halperin S, Pelletier L, Talbot J. Invasive pneumococcal infections in Canadian children, 1991–1998: implications for new vaccination strategies. Canadian Paediatric Society/Laboratory Centre for Disease Control Immunization Monitoring Program, Active (IMPACT). Clin Infect Dis. 2000;31(1):58–64.
Bank of Canada. Inflation calculator. 2017. https://www.bankofcanada.ca/rates/related/inflation-calculator/. Accessed 6 Oct 2017.
Statistics Canada. Table 282–0087 and Catalogue no. 71–001-XIE. Labour force characteristics, seasonally adjusted, by province (monthly) (Quebec, Ontario, Manitoba). 2016. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1410028703. Accessed 6 Oct 2017.
Statistics Canada. Table 282–0167—labour force survey estimates (LFS), average usual hours and wages of employees by age group, sex, union coverage, job permanency, and National Occupational Classification (NOC), unadjusted for seasonality, monthly (persons unless otherwise noted), CANSIM (database). 2016. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1410032001. Accessed 6 Oct 2017.
Lau WC, Murray M, El-Turki A, Saxena S, Ladhani S, Long P, et al. Impact of pneumococcal conjugate vaccines on childhood otitis media in the United Kingdom. Vaccine. 2015;33(39):5072–9.
De Wals P, Carbon M, Sevin E, Deceuninck G, Ouakki M. Reduced physician claims for otitis media after implementation of pneumococcal conjugate vaccine program in the province of Quebec, Canada. Pediatr Infect Dis J. 2009;28(9):e271–5.
Dagan R, Pelton S, Bakaletz L, Cohen R. Prevention of early episodes of otitis media by pneumococcal vaccines might reduce progression to complex disease. Lancet Infect Dis. 2016;16(4):480–92.
McIntyre PB, Berkey CS, King SM, Schaad UB, Kilpi T, Kanra GY, et al. Dexamethasone as adjunctive therapy in bacterial meningitis. A meta-analysis of randomized clinical trials since 1988. JAMA. 1997;278(11):925–31.
Pomeroy SL, Holmes SJ, Dodge PR, Feigin RD. Seizures and other neurologic sequelae of bacterial meningitis in children. N Engl J Med. 1990;323(24):1651–7.
Wilson M, Wasserman M, Breton M, Peloquin F, McDade C, Earnshaw S, et al, editors. Potential clinical and economic impact of switching from the 13-valent to 10-valent pneumococcal conjugate vaccine in Quebec. In: Canadian immunization conference 2016; Ottawa, Ontario, Canada. 2016.
Wasserman M, Wilson M, Breton M, Peloquin F, McDade C, Farkouh R, editors. Estimating the clinical and economic impact of switching from the 13-valent pneumococcal conjugate vaccine (PCV13) to a lower-valent (PCV10) vaccine in Canada. In: 35th annual meeting of the European society for paediatric infectious diseases. Madrid, Spain; 2017.
De Wals P, Nguyen VH, Erickson LJ, Guay M, Drapeau J, St-Laurent J. Cost-effectiveness of immunization strategies for the control of serogroup C meningococcal disease. Vaccine. 2004;22(9–10):1233–40.
Statistics Canada. Table 282–0225—labour force survey estimates (LFS), average weekly earnings, average hourly wage rate and average usual weekly hours by union status and type of work, Canada and provinces, annual. 2016. https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1410013401. Accessed 6 Oct 2017.
World Health Organization. WHO guide for standardization of economic evaluations of immunization programmes. Department of Immunization, Vaccines and Biologicals; 2008. http://apps.who.int/iris/bitstream/handle/10665/69981/WHO_IVB_08.14_eng.pdf;jsessionid=B691B7CFC11EEADBF9632384CB4C5B92?sequence=1. Accessed 1 Dec 2016.
Bennett JE, Sumner W 2nd, Downs SM, Jaffe DM. Parents’ utilities for outcomes of occult bacteremia. Arch Pediatr Adolesc Med. 2000;154(1):43–8.
Cheng AK, Niparko JK. Cost-utility of the cochlear implant in adults: a meta-analysis. Arch Otolaryngol Head Neck Surg. 1999;125(11):1214–8.
National Institute for Health and Welfare. Incidence of invasive pneumococcal disease in Finland. 2017. https://thl.fi/fi/web/thlfi-en/research-and-expertwork/projects-and-programmes/monitoring-the-population-effectiveness-of-pneumococcal-conjugate-vaccination-in-the-finnish-national-vaccination-programme/incidence-of-invasive-pneumococcal-disease-in-finland. Accessed 1 Dec 2017.
Knol MJ, de Melker HE, Sanders EAM, van der Ende A. Incidence of IPD in The Netherlands up to 5 years after introduction of PCV10. In: 10th International Symposium on Pneumococci and Pneumococcal Diseases; Glasgow, United Kingdom. 2016.
Moore MR, Link-Gelles R, Schaffner W, Lynfield R, Lexau C, Bennett NM, et al. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet Infect Dis. 2015;15(3):301–9.
Waight PA, Andrews NJ, Ladhani SN, Sheppard CL, Slack MP, Miller E. Effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: an observational cohort study. Lancet Infect Dis. 2015;15(5):535–43.
Collins S, Sheppard C, Lit tD, Fry NK, Andrews N, Miller E, et al. Trends in invasive pneumococcal disease over time: England and Wales 2000/01 to 2014/15. 10th International Symposium on Pneumococci and Pneumococcal Diseases; Glasgow, Scotland. 2016.
Ladhani SN, Collins S, Djennad A, Sheppard CL, Borrow R, Fry NK, et al. Rapid increase in non-vaccine serotypes causing invasive pneumococcal disease in England and Wales, 2000–17: a prospective national observational cohort study. Lancet Infect Dis. 2018;18(4):441–51.
Knol M, Sanders EAM, de Melker H. Pneumococcal disease in The Netherlands: background information for the Health Council of The Netherlands. 2017. https://www.rivm.nl/dsresource?objectid=3dcf9b97-d7f4-41e8-8b13-cb43a182a7ea&type=pdf&disposition=inline. Accessed 10 Jan 2018.
Tagarro A, Benito A, Sanchez A, Aznar E, Otheo E, Sanz-Rosa D. Bacteremic pneumonia before and after withdrawal of 13-valent pneumococcal conjugate vaccine from a public vaccination program in Spain: a case-control study. J Pediatr. 2016;171:111–5 (e1–3).
Picazo J, Ruiz-Contreras J, Casado-Flores J, Negreira S, Baquero F, Hernandez-Sampelayo T, et al. Effect of the different 13-valent pneumococcal conjugate vaccination uptakes on the invasive pneumococcal disease in children: analysis of a hospital-based and population-based surveillance study in Madrid, Spain, 2007–2015. PLoS One. 2017;12(2):e0172222.
Waye A, Chuck AW. Value added by the Prevnar 13 Childhood Immunization Program in Alberta, Canada (2010–2015). Drugs. 2015;2(3):311–8.
Knerer G, Ismaila A, Pearce D. Health and economic impact of PHiD-CV in Canada and the UK: a Markov modelling exercise. J Med Econ. 2012;15(1):61–76.
Klok RM, Lindkvist RM, Ekelund M, Farkouh RA, Strutton DR. Cost-effectiveness of a 10- versus 13-valent pneumococcal conjugate vaccine in Denmark and Sweden. Clin Ther. 2013;35(2):119–34.
Shiragami M, Mizukami A, Leeuwenkamp O, Mrkvan T, Delgleize E, Kurono Y, et al. Reply to Farkouh RA et al. Comment on “Cost-Effectiveness Evaluation of the 10-Valent Pneumococcal Non-Typeable Haemophilus Influenzae Protein D Conjugate Vaccine and 13-Valent Pneumococcal Vaccine in Japanese Children”. Infect Dis Therapy. 2015;4(2):235–44.
Delgleize E, Leeuwenkamp O, Theodorou E, Van de Velde N. Cost-effectiveness analysis of routine pneumococcal vaccination in the UK: a comparison of the PHiD-CV vaccine and the PCV-13 vaccine using a Markov model. BMJ Open. 2016;6(11):e010776.
Gomez JA, Villasenor-Sierra A, Aguilar GM, Manjarrez RC, Cervantes-Apolinar MY. Estimacion de la relacion costo-efectividad de las vacunas neumococicas conjugadas prevenar-13 y Synflorix(R), utilizadas en los programas de vacunacion de poblacion infantil Mexicana. Value Health Regional Issues. 2016;11:76–84.
Zhou Z, Deceuninck G, Lefebvre B, De Wals P. Forecasting trends in invasive pneumococcal disease among elderly adults in Quebec. Can J Infect Dis Med Microbiol. 2017;2017:4347206.
Isturiz R, Sings HL, Hilton B, Arguedas A, Reinert RR, Jodar L. Streptococcus pneumoniae serotype 19A: worldwide epidemiology. Exp Rev Vaccines. 2017;16(10):1007–27.
Rinta-Kokko H, Palmu AA, Auranen K, Nuorti JP, Toropainen M, Siira L, et al. Long-term impact of 10-valent pneumococcal conjugate vaccination on invasive pneumococcal disease among children in Finland. Vaccine. 2018;36(15):1934–40.
Brandileone MC, Almeida SCG, Minamisava R, Andrade AL. Distribution of invasive Streptococcus pneumoniae serotypes before and 5 years after the introduction of 10-valent pneumococcal conjugate vaccine in Brazil. Vaccine. 2018;36(19):2559–66.
DeWals ea, editor Impact of pneumococcal 23-valent polysaccharide (PPV23) and 13-valent conjugate vaccine (PCV13) on invasive pneumococcal diseases (IPD) caused by serotype 3 (ST3). In: 10th World congress of the world society for pediatric infectious diseases; Shenzhen, China 2017.
Kuhlmann A, von der Schulenburg JG. Modeling the cost-effectiveness of infant vaccination with pneumococcal conjugate vaccines in Germany. Eur J Health Econ. 2017;18(3):273–92.
Kulpeng W, Sornsrivichai V, Chongsuvivatwong V, Rattanavipapong W, Leelahavarong P, Cairns J, et al. Variation of health-related quality of life assessed by caregivers and patients affected by severe childhood infections. BMC Pediatrics. 2013;13:122.
van Hoek AJ, Choi YH, Trotter C, Miller E, Jit M. The cost-effectiveness of a 13-valent pneumococcal conjugate vaccination for infants in England. Vaccine. 2012;30(50):7205–13.
Prymula R, Kriz P, Kaliskova E, Pascal T, Poolman J, Schuerman L. Effect of vaccination with pneumococcal capsular polysaccharides conjugated to Haemophilus influenzae-derived protein D on nasopharyngeal carriage of Streptococcus pneumoniae and H. influenzae in children under 2 years of age. Vaccine. 2009;28(1):71–8.
Prymula R, Peeters P, Chrobok V, Kriz P, Novakova E, Kaliskova E, et al. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: a randomised double-blind efficacy study. Lancet (London, England). 2006;367(9512):740–8.
Wang XJ, Saha A, Zhang X. Cost-effectiveness analysis of a universal mass vaccination program with a PHiD-CV 2 + 1 schedule in Malaysia. Cost Effect Res Alloc. 2017;15(1):17.
Castiglia P, Pradelli L, Castagna S, Freguglia V, Palu G, Esposito S. Overall effectiveness of pneumococcal conjugate vaccines: an economic analysis of PHiD-CV and PCV-13 in the immunization of infants in Italy. Hum Vaccines Immunother. 2017;13(10):2307–15.
Pastor L, Sings H, Hilton B, Kohli M, Kruse M, Wasserman M, et al, editors. A systematic review of pneumococcal conjugate vaccine (PCV) impact on acute otitis media (OM) and nasopharyngeal carriage (NP) due to nontypeable haemophilus influenza (NTHi). In: 35th annual meeting of the European society for paediatric infectious diseases; Madrid, Spain. 2017.
Desai S, Policarpio ME, Wong K, Gubbay J, Fediurek J, Deeks S. The epidemiology of invasive pneumococcal disease in older adults from 2007 to 2014 in Ontario, Canada: a population-based study. CMAJ Open. 2016;4(3):E545–50.
Public Health Ontario. Reportable Disease Trends in Ontario, 2014. Toronto, ON: Ontario Agency for Health Protection and Promotion (Public Health Ontario); 2016. http://www.publichealthontario.ca/en/eRepository/Reportable_Disease_Trends_in_Ontario_2014.pdf. Accessed 11 Aug 2017.
Mangen MJ, Huijts SM, Bonten MJ, de Wit GA. The impact of community-acquired pneumonia on the health-related quality-of-life in elderly. BMC Infect Dis. 2017;17(1):208.
Andrade LF, Saba G, Ricard JD, Messika J, Gaillat J, Bonnin P, et al. Health related quality of life in patients with community-acquired pneumococcal pneumonia in France. Health Qual Life Outcomes. 2018;16(1):28.
Acknowledgements
Funding
This study was conducted by RTI Health Solutions, Research Triangle Park, NC, under the direction of Pfizer, Inc., and was funded by Pfizer, Inc., New York, NY, which owns Prevnar 13. Pfizer provided funding for the development of the model and manuscript as well as for article processing charges. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis.
Medical Writing, Editorial, and Other Assistance
We acknowledge Dr. Alison McGeer for providing us with the Toronto Invasive Bacterial Disease Network serotype-specific IPD surveillance data and allowing their use for our modeling research. These data were instrumental in our analyses.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
This work was sponsored by Pfizer Inc. Michele Wilson is an employee of RTI Health Solutions, who were paid consultants to Pfizer in connection with the development of this manuscript. Cheryl McDade is an employee of RTI Health Solutions, who were paid consultants to Pfizer in connection with the development of this manuscript. Stephanie Earnshaw is an employee of RTI Health Solutions, who were paid consultants to Pfizer in connection with the development of this manuscript. Maarten Postma was a paid consultant to Pfizer (or received an honorarium from Pfizer) in connection with the development of this manuscript. Matt Wasserman is an employee of Pfizer, Inc., who provided funding for the development of this manuscript. Marie-Claude Breton is an employee of Pfizer, Inc., who provided funding for the development of this manuscript. Francois Peloquin is an employee of Pfizer, Inc., who provided funding for the development of this manuscript. Heather Sings is an employee of Pfizer, Inc., who provided funding for the development of this manuscript. Raymond Farkouh is an employee of Pfizer, Inc., who provided funding for the development of this manuscript. Taj Jadavi has nothing to disclose.
Compliance with Ethics Guidelines
This article does not involve the study of human participants or animals performed by any of the authors and as such was not subject to institutional review board approval.
Data Availability
This study did not utilize any publically available data sets.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced digital features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.6446075.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Wilson, M., Wasserman, M., Jadavi, T. et al. Clinical and Economic Impact of a Potential Switch from 13-Valent to 10-Valent Pneumococcal Conjugate Infant Vaccination in Canada. Infect Dis Ther 7, 353–371 (2018). https://doi.org/10.1007/s40121-018-0206-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40121-018-0206-1