Abstract
Synthesized poly(2,6-diphenyl-p-phenylene oxide) (PPPOdp) and brominated poly(2,6-diphenyl-p-phenylene oxide) (BPPPOdp) are identified as new membranes for CO2/N2 separation and characterized by SEM, FTIR, and 1HNMR. BPPPOdp is known as a flexible membrane with higher permselectivity (\(\alpha_{{{\text{CO}}_{ 2} / {\text{N}}_{ 2} }}\) = 30.53) than PPPOdp membranes. BPPPO and SiO2 nanocomposite membranes display improved CO2 permeability and CO2/N2 selectivity compared to the pure PPPOdp, and BPPPOdp membranes. The CO2/N2 separation mechanism in the membranes is related to the amount of gas dissolution than the amount of gas diffusion.
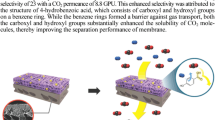
Graphical abstract

Similar content being viewed by others
Introduction
One of the straightest techniques to decrease the global warming effect due to atmospheric CO2 increase is found in CO2 removal from gaseous streams which is produced in the production of energy from industrial processes [1, 2]. Using membrane technology in gas separation has created great revolution compared to traditional separation processes [3, 4].That is owing to their low cost, excellent efficiency and being trustworthy.
Recently, three popular gas separation membranes including polymeric membrane, inorganic membrane and new block polymeric membrane are used extensively for CO2 separation. However, all of them have some defects to industrialization [5]. Highly permeable inorganic membranes suffer from brittle structure, structure defects and also high costs. Therefore, preparation, characterization, and application of inorganic separation membranes were just limited to the laboratory. While, polymeric separation membranes attract more and more attention because of the no expensive and easily fabricated materials. The modified polymeric membranes display improved permeability and CO2 selectivity. Polymeric membranes are commonly used for CO2 separation because of their extensive applications: separation of CO2/H2 in gas refinement, separation of CO2/N2 in carbon capture, separation of CO2/CH4 in natural gas refinement, separation of CO2/O2 in food packaging [6].
The mechanism of gas separation is distinguished by size sieving because of the rigid chain structures of the polymer matrix [7]. Polymeric membranes with great permeability and selectivity are difficult to prepare.
Poly(2,6-dimethyl-p-phenyleneoxide) (PPPOdm) as a glassy polymeric material is introduced suitably as a gas separation membrane, because of the great permeability and suitable selectivity [1]. In particular, PPPOdm membrane with great CO2 permeability (~ 40 bar) and great CO2/N2 selectivity (~ 15) is preferred to other glassy polymers [8]. Chemically modified membranes such as brominated poly(2,6-dimethyl-p-phenyleneoxide) (BPPPOdm) could often improve the gas permeability and selectivity [9, 10].
Addition of nanosized fillers to the polymer matrix could improve the separation performance of polymeric materials through modifying the chain packing of the polymer [1, 11]. Nano-scaled fillers like to be adjusted to the chain packing of glassy polymers in a way that increases the permselectivity trough creating additional selective free holes.
The SiO2, TiO2 and MoS2 nanoparticles through polymer chain interruption bring improvement in gas separation. Polymeric membrane with dispersing nanoparticles is usually challenging due to undesirable interactions between the polymeric matrix and inorganic nano fillers. The appropriate polymer selection and applying the useful inorganic nanoparticle are two essential issues to get useful polymeric membranes [12].
Among different inorganic nanoparticles, SiO2 nanoparticles are identified as an appropriate modifier for gas separation membranes owing to their high specific surface area and the ability of chemical functionalization, high mechanical and thermal stabilities [12,13,14,15,16].
Merkel et al. introduced poly(4-methyl-2-pentyne)(PMP)/silica nanocomposite including silica NPs (30 wt%), as more effective membrane than pure PMP at gas separation [17]. Also poly(amide-6-b-ethylene oxide) (PEBAX) including 27 wt% silica NPs displayed 277 bar for CO2 permeability of and 79 for \(\alpha_{{{\text{CO}}_{ 2} / {\text{N}}_{ 2} }}\) selectivity [18].
With regard to the previous literature, here, we synthesized poly(2,6-diphenyl-p-phenylene oxide) (PPPO), brominated poly(2,6-diphenyl-p-phenylene oxide) (BPPPO), and BPPPO/SiO2 nanocomposite as suitable porous membrane for CO2 adsorption (Fig. 1).
Experimental
Materials
PPOdm (Mn 25,000, polydispersity 2.0), N,N,Nʹ,Nʹ tetramethylethylenediamine (TMEDA, 99%), 2,6-diphenylphenol (98%), bromine (Br2, 99.5%), chloroform (CHCl3, 99.8%), ethanol (99.8%), methanol (99.8%), anhydrous hydrazine (98%), 1,2-dichlorobenzene (98.5%), chlorosulfonic acid (99%), anhydrous magnesium sulfate (99.5%), glacial acetic acid (99.8%) and silica nanopowders (SiO2, 99.5%, 10 nm) and copper (I) chloride (CuCl, 98.5%) from Aldrich.
Synthesis of PPPOdp
The synthesis of PPPOdp was performed according to reported procedure [19]. In a 100 cc flask, CuCl (0.041 g), TMEDA (0.031 g), and anhydrous magnesium sulfate (2 g) are added to 1,2-dichlorobenzene (35 cc). The mixture is heated in an oil bath at 65 °C under magnetic stirring. Oxygen is bubbled into the mixture for 15 min. After green color observation in solution, 40 cc of 2,6-diphenylphenol solution is added dropwise during 20 min and made a dark red color immediately. After 24 h, several drops of anhydrous hydrazine are added to the solution to remove byproducts. The inorganic solids are filtered, and the solution is added dropwise to methanol (400 cc) including hydrazine and the solution is shaken for several hours. Then produced polymer is filtered and resolved in chloroform (40 cc) and precipitated in methanol (400 cc). The 3 g of polymer with Mn ∼ 120,000 is collected by filtration and dried at 80 °C under vacuum for 24 h. The density of PPPO is ∼ 1.11 g cm−3 measured by an excluded volume method at room temperature.
Synthesis of BPPPOdp
For synthesis of BPPPO, PPPO (5 g) and CHCl3 (50 cc) are added to a 100 cc flask. The mixture is stirred with a magnetic stirrer. The 20 cc of bromine solution included Br2 (10 cc) and CHCl3 (10 cc), is added dropwise to the mixture during 30 min under Argon atmosphere. After 1 h stirring at room temperature, 800 cc ethanol is added under mechanical stirring and the BPPPO is precipitated out. The produced polymer is filtered and dried at room temperature under vacuum. The extent of bromination is 80–95% from NMR spectrum. The density of BPPPO is ∼ 1.43 g cm−3 measured by the excluded volume method at room temperature with Mn ∼ 180,000.
Membrane preparation
The PPPOdp, BPPOdp, and BPPPOdp/SiO2 nanocomposite membranes are fabricated. The solution of PPPO (3 wt%, in CHCl3) is cast on glass plate at room temperature. Similarly, the BPPPO membrane is prepared.
The BPPPO/silica membrane is prepared as follows: 0.3 g BPPPO is dissolved in 5 cc CHCl3 under stirring. Silica NPs (10 nm) is added slowly to the BPPPO solution. The mixture is vigorously stirred with 1150 rpm for 15 min to dispersion. Then mixture is cast onto a plate at room temperature to dry. After drying, the resulting membranes, with 55–85 µm thickness, are peeled off and stored in a desiccator for testing.
Adding nanoparticles to glassy polymer membranes increases the gas permeability through disturbed polymer chain packing and then increase free volume of membranes.
Gas separation experiments
The membranes gas permeability is examined using a constant-volume setup (Fig. 2). The test setup is made of two compartment stainless steel cells in a way that a flat circular membrane with an effective area of 10.45 cm2, and a reticular steel support are placed between two flanges and held by O-rings to avoid gas leakage. The experiments are started by vacating the cell up to − 0.1 bar for sending out any remaining gases before using feeding gases, which is to avoid further measurement errors. The rate of permeation (Barrer) is calculated from the pressure changes on the permeate side versus time, according to the following equation (1 Barrer = 10−10 cm3 (STP)-cm/cm2 s cmHg):
where V refers to the volume of the permeate side in cm, L and A represent membrane thickness (cm) and the effective area (cm2), and T is the absolute temperature. p2 is the feed pressure of the upstream in bar and dp/dt is the slope of permeate pressure change versus time, in its steady state (linear) region.
Permeability data of CO2 and N2 is measured for three times, and the average values are reported. The membranes CO2/N2 ideal selectivity (\(\alpha_{{{\text{CO}}_{ 2} / {\text{N}}_{ 2} }}\)) is determined as the ration of CO2 permeation to N2 permeation using the following equation: \(\alpha = P_{{{\text{CO}}_{ 2} }} /P_{{{\text{N}}_{ 2} }}\); where \({\text{P}}_{{{\text{CO}}_{ 2} }}\) and PB are related to the permeabilities of pure CO2 and N2, respectively [20,21,22].
Characterization
Scanning electron microscope (SEM) (Philips 505) is used to detect the morphology and particle dispersion in all three membranes. The SEM is used at 10 kV operation voltage. FTIR spectra of pure PPPOdp, and BPPOdp are obtained with a FTIR spectrometer (Thermoscientific Nicolet 6700) in the range of 4000–400 cm−1. The solution of PPPOdp, and BPPOdp in CDCl3 (~ 2% w) are used for 1HNMR analyses on a Bruker Advance DRX-400 spectrometer.
Result and discussion
Characterization of PPPO, BPPPO, and BPPPO/SiO2 nanocomposite and their membranes
Differential scanning calorimetry (DSC)
Thermal properties of the white crystalline PPPOdp, and yellowish crystalline BPPPOdp are investigated by DSC analysis (Table 1). To eliminate the influences of less favorable crystal phases are formed at 248 °C, 288 °C for PPPO, BPPPO, respectively. Higher Tg of BPPPOdp is related to three bulky bromine groups in each monomer unit. The second heating run as their melting point are considered at 470 and > 500 °C for PPPO and BPPPO that confirm their crystalline structures.

The scanning electron microscope image (SEM)
The gas transport properties strongly depend on the membranes morphology. The cross section morphology of the neat PPPOdp, BPPPOdp, and BPPPOdp/silica nanocomposite membranes are investigated via SEM images (Fig. 3). Figure 3a show porous structure for PPPOdp membrane while Fig. 3b displays optimized porous structure with fewer surface defects for its relatively brominated membrane (BPPPOdp). Adding silica nanoparticles to the brominated membrane (BPPPOdp) propel it to an agglomerated BPPPOdp/silica nanocomposite membrane (Fig. 3c). The nanocomposite membranes with their hydrophilic characterization of silica nanoparticles prefer to agglomerate. Hence BPPPOdp/silica nanocomposite membrane seems to appear more selective due to its high density.
FT-IR
Functional groups on the surface of poly(2,6-diphenyl-p-phenylene oxide) PPPOdp and brominated poly(2,6-diphenyl-p-phenylene oxide) (BPPPOdp) are investigated by means of FTIR spectra (Fig. 4). The observed peaks at 1400–1600 cm−1 are assigned to the C–C stretching bands of the aromatic rings. PPPOdp reveals that some of the CH stretching of aromatic rings at 3025 cm−1 (Fig. 4a). In addition, the observed peaks at 695 cm−1, and 1147 cm−1 corresponds to out of plane bending aromatic CH and C–O stretching bond.
As mentioned before, the observed peaks at 1400, 1600 cm−1 illustrate aromatic CC stretching, 3058 cm−1 clarify aromatic CH stretching, and 1183 cm−1 show out of plane bending aromatic CH bond for brominated poly(2,6-diphenyl-p-phenylene oxide) (BPPPOdp). Also, monitored peaks around 1030–1050 and 1183 cm−1 imply C–Br and C–O stretching bond, respectively.
Nuclear magnetic resonance (NMR)
In 1HNMR spectrum of poly(2,6-diphenyl-p-phenylene oxide) (PPPOdp) seen some peaks at 6.28 (2H, C6H2O), 6.96, 7.1–7.3 ppm (10H, 2 C6H5) (Fig. 5a). While, brominated poly(2,6-diphenyl-p-phenylene oxide) (BPPPOdp) demonstrate some peaks at 6.32 (1H, C6HOBr), 6.75 and 7.4–7.8 ppm (8H, 2 C6H4Br) (Fig. 5b). As seen, decrease of the number of hydrogen atoms in brominated poly(2,6-diphenyl-p-phenylene oxide) (BPPPOdp) could confirm the new structure from brominating (Fig. 5b).
Gas separation performance test
Three prepared membranes of PPPOdp, BPPPOdp, BPPPOdp/SiO2 nanocomposite are used in the gas separation test (Table 2). According to previous works, the permeability of pure gas is measured in a constant-volume variable-pressure unit and the permselectivity (α) is determined from the following formula: α = PA/PB; where PA and PB are related to the permeabilities of pure gases A and B, respectively (Fig. 2, Table 2) [20,21,22].
The brominated poly(2,6-dimethyl-1,4-phenylene oxide) (BPPPOdp) flexible membrane is identified with lower permeability of CO2 (\({\text{P}}_{{{\text{CO}}_{ 2} }}\) = 58 Barrer) but higher selectivity (\(\alpha_{{{\text{CO}}_{ 2} / {\text{N}}_{ 2} }}\) = 30.53) than poly(2,6-dimethyl-1,4-phenylene oxide) (PPPOdm) membranes with permeability of CO2 (\({\text{P}}_{{{\text{CO}}_{ 2} }}\) = 68 Barrer) and selectivity (\(\alpha_{{{\text{CO}}_{ 2} / {\text{N}}_{ 2} }}\) = 14.7) (Table 2, Fig. 6). Thus, BPPPOdp is selected as a beginning material for manufacture process of polymer/SiO2 nanocomposite membrane. The BPPPOdp/SiO2 nanocomposite membrane including 10 wt% silica NPs present a greatly enhanced CO2 permeability in (\({\text{P}}_{{{\text{CO}}_{ 2} }}\) = 85 Barrer) and (\(\alpha_{{{\text{CO}}_{ 2} / {\text{N}}_{ 2} }}\) = 40.48). Hence they are found more effective than poly(4-methyl-2-pentyne)(PMP)/silica nanocomposite with permeability and selectivity for large organic molecules [17]. Meanwhile poly(amide-6-b-ethylene oxide)/silica nanocomposite displayed 277 bar permeability of CO2 and \(\alpha_{{{\text{CO}}_{ 2} / {\text{N}}_{ 2} }}\) = 79 [18].
The permselectivity diagram (\(\alpha_{{{\text{CO}}_{ 2} / {\text{N}}_{ 2} }}\)) of poly(2,6-diphenyl-p-phenylene oxide) (PPPOdp) (blue), brominated poly(2,6-diphenyl-p-phenylene oxide) (BPPPOdp) (orange), and BPPPOdp/SiO2 nanocomposite membranes (gray) (according to the calculation in Table 2)
The study of mechanism demonstrates the silica nanoparticles owing to their incompatibility with polymer chains produce some gaps in the structure of nanocomposite, which results in enhanced permeability [19]. The surface of silica NPs with polar silano groups displayed low compatibility with the BPPOdp matrix that causes silica particles to aggregate in the membrane. A narrow gap around the silica NPs formed due to lack of tight interaction between polymer chains and silica NPs, which made high gas diffusivity and permeability (Fig. 7).
Conclusion
Poly(2,6-diphenyl-p-phenylene oxide) (PPPOdp), brominated poly(2,6-diphenyl-p-phenylene oxide) (BPPPOdp), and BPPPOdp/SiO2 nanocomposite are synthesized and compared together in CO2 permeability and CO2/N2 permselectivity. BPPPOdp membranes are known to be more efficient than PPPOdp through higher CO2/N2 permselectivity. The BPPPOdp/SiO2 nanocomposite membrane with the highest CO2 permeability and CO2/N2 permselectivity is introduced as the most effective membrane. The CO2 permeability increase through silica addition to the membrane.
References
Rea, R., Ligi, S., Christian, M., Morandi, V., Giacinti Baschetti, M., De Angelis, M.: Permeability and selectivity of PPO/graphene composites as mixed matrix membranes for CO2 capture and gas separation. Polymers. 10, 129–148 (2018)
Bains, P., Psarras, P., Wilcox, J.: CO2 capture from the industry sector. Prog. Energy Combust. Sci. 63, 146–172 (2017)
George, G., Bhoria, N., AlHallaq, S., Abdala, A., Mittal, V.: Polymer membranes for acid gas removal from natural gas. Sep. Purif. Technol. 158, 333–356 (2016)
Hou, J., Zhang, H., Hu, Y., Li, X., Chen, X., Kim, S., Wang, Y., Simon, G.P., Wang, H.: Carbon nanotube networks as nanoscaffolds for fabricating ultrathin carbon molecular sieve membranes. ACS Appl. Mater. Interfaces. 10, 20182–20188 (2018)
Li, H., Ding, X., Zhang, Y., Liu, J.: Porous graphene nanosheets functionalized thin film nanocomposite membrane prepared by interfacial polymerization for CO2/N2 separation. J. Memb. Sci. 543, 58–68 (2017)
Sodeifian, G., Raji, M., Asghari, M., Rezakazemi, M., Dashti, A.: Polyurethane-SAPO-34 mixed matrix membrane for CO2/CH4 and CO2/N2 separation. Chin. J. Chem. Eng. (2018). https://doi.org/10.1016/j.cjche.2018.03.012
Wang, Y., Li, H., Dong, G., Scholes, C., Chen, V.: Effect of fabrication and operation conditions on CO2 separation performance of PEO–PA block copolymer membranes. Ind. Eng. Chem. Res. 54, 7273–7283 (2015)
Sridhar, S., Smitha, B., Ramakrishna, M., Aminabhavi, T.M.: Modified poly(phenylene oxide) membranes for the separation of carbon dioxide from methane. J. Memb. Sci. 280, 202–209 (2006)
Hamad, F., Matsuura, T.: Performance of gas separation membranes made from sulfonated brominated high molecular weight poly(2,4-dimethyl-l,6-phenylene oxide). J. Memb. Sci. 253, 183–189 (2005)
Cong, H., Yu, B., Tang, J., Zhao, X.S.: Ionic liquid modified poly(2,6-dimethyl-1,4-phenylene oxide) for CO2 separation. J. Polym. Res. 19, 9761–9769 (2012)
Chu, L., Deng, S., Zhao, R., Deng, J., Kang, X.: Comparison of adsorption/desorption of volatile organic compounds (VOCs) on electrospun nanofibers with tenax TA for potential application in sampling. PLoS One 11(10), e0163388 (2016)
Aghaei, Z., Naji, L., Hadadi Asl, V., Khanbabaei, G., Dezhagah, F.: The influence of fumed silica content and particle size in poly (amide 6-b-ethylene oxide) mixed matrix membranes for gas separation. Sep. Purif. Technol. 199, 47–56 (2018)
Zargar, V., Asghari, M., Afsari, M.: Gas separation properties of swelled nanocomposite chitosan membranes cross-linked by 3-aminopropyltriethoxysilane. Int. J. Environ. Sci. Technol. (2017). https://doi.org/10.1007/s13762-017-1554-1
Ghaee, A., Ghadimi, A., Sadatnia, B., Ismail, A.F., Mansourpour, Z., Khosravi, M.: Synthesis and characterization of poly(vinylidene fluoride) membrane containing hydrophobic silica nanoparticles for CO2 absorption from CO2/N2 using membrane contactor. Chem. Eng. Res. Des. 120, 47–57 (2017)
Isanejad, M., Mohammadi, T.: Effect of amine modification on morphology and performance of poly (ether-block-amide)/fumed silica nanocomposite membranes for CO2/CH4 separation. Mater. Chem. Phys. 205, 303–314 (2018)
Ghadimi, A., Mohammadi, T., Kasiri, N.: Gas permeation, sorption and diffusion through PEBA/SiO2 nanocomposite membranes (chemical surface modification of nanoparticles). Int. J. Hydrogen Energy 40, 9723–9732 (2015)
Merkel, T.C., Freeman, B.D., Spontak, R.J., He, Z., Pinnau, I., Meakin, P., Hill, A.J.: Ultrapermeable, reverse-selective nanocomposite membranes. Science. 296, 519–522 (2002)
Kim, J.H., Lee, Y.M.: Gas permeation properties of poly(amide-6-b-ethylene oxide)–silica hybrid membranes. J. Memb. Sci. 193, 209–225 (2001)
Yu, B., Cong, H., Zhao, X.: Hybrid brominated sulfonated poly(2,6-diphenyl-1,4-phenylene oxide) and SiO2 nanocomposite membranes for CO2/N2 separation. Proc. Natl. Sci. Mater. 22, 661–667 (2012)
Cong, H., Yu, B.: Aminosilane cross-linked PEG/PEPEG/PPEPG membranes for CO2/N2 and CO2/H2 separation. Ind. Eng. Chem. Res. 49, 9363–9369 (2010)
Koros, W.J., Chan, A.H., Paul, D.R.: Sorption and transport of various gases in polycarbonate. J. Memb. Sci. 2, 165–190 (1977)
Felder, R.M., Huvard, G.S.: 17. Permeation, diffusion, and sorption of gases and vapors. In: Fava, R.A. (ed.) Methods in Experimental Physics, vol. 16, pp. 315–377. Academic, New York (1980)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ghanbarian, H., Mirza, B. Synthesis and characterization of brominated poly(2,6-diphenyl-p-phenylene oxide)/SiO2 nanocomposite membrane for CO2/N2 separation. J Nanostruct Chem 8, 413–420 (2018). https://doi.org/10.1007/s40097-018-0274-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40097-018-0274-5