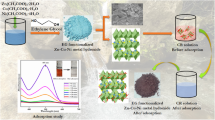
Abstract
Zn0.3Fe0.45O3 bimetallic oxide nanoparticle (ZnFeBONp) was synthesized and characterized by FESEM, EDS, XRD, BET, TEM and FT-IR techniques with the aim of exploring its application for removing of Congo red dye from waste samples. The effects of pH, contact time, adsorbent dosage and dye concentration on the removal of dye were investigated and optimized as pH 6.5, 40 min contact time, 0.2 g adsorbent dose for 20 ppm dye. Results indicated that the synthesized adsorbent could effectively remove high concentrations of dye in a short contact time. Isotherm modeling revealed that the Langmuir isotherm could better describe the adsorption of the dye on the ZnFeBONp as compared to other models. The qmax up to 333.33 mg g−1. The results showed that the adsorption system followed the Ho and McKay equations for the entire adsorption. Kinetics of Congo red adsorption on ZnFeBONp best fit with the pseudo-second-order model. Because of the high-specific surface area and nano-scale particle size, ZnFeBONp indicated favorable adsorption behavior for dye.
Similar content being viewed by others
Introduction
Water pollution due to industrial dye effluent is a very serious problem which undergoes chemical as well as biological changes, consume dissolved oxygen, and destroy aquatic life [1,2,3]. Dyes are used in various industries such as construction, textile, paper, plastics, leather, cosmetics, etc., for the purpose of coloring the related products. Therefore, large amount of colored wastewater is produced. Many dyes are highly poisonous, carcinogenic, and stable from daylight and oxidation. Dyes not only make water colorful but also do harm for the survival of aquatic life and the ecosystem [2]. For example, dyes will deadly affect the photosynthetic aquatic life due to the reduction of light penetration [3]. Thus, the researcher working for developing an effective and suitable way to eliminate dye contamination from wastewater has become an urgent issue.
Till date, several techniques, such as coagulation, biological treatment, chemical oxidation, photocatalytic degradation and adsorption, have been explored to remove the dye contaminants from wastewater [4,5,6,7]. Adsorption is a more competitive method for removal of dyes due to its high efficiency and economy. Currently, adsorption has been proved to be a simple and time-saving technology for removal of dyes [8], in which the key technology is to exploit selective and efficient adsorbents [9, 10]. Conventional adsorbents such as silica gel [11], activated alumina [12] and zeolite molecular sieve [13, 14], displayed low removal efficiency.
In last few years, we synthesized the organic receptor for the selective and sensitive detection of metal ion and removal of phosphate from waste water [15,16,17,18,19,20,21,22,23]. In the current study, we report the synthesis of ZnFeBONP and explore its ability to remove Congo red (CR) from aqueous solution. Congo red was taken as models for azo dyes, for removal investigation. For the present study, a batch-contact-time method was used, and the equilibrium of CR adsorption on to ZnFeBONP was investigated with attempts to fit the data to Langmuir, Freundlich and Temkin equations. The uptake of CR on ZnFeBONP was examined as a function of adsorbate concentration, adsorbent concentration and contact time. The kinetic order and thermodynamic parameter was deduced on CR adsorption on ZnFeBONP (Scheme 1).
Materials and method
Instrumentation
A Metrohm model 713 pH-meter was used for pH measurements. Field emission scanning electron microscopy (FE-SEM) images were obtained with a Bruker S-4800 instrument operated at 15.0 kV. EDX was done on the same instrument at PM Image size: 500 × 375 Mag:40,000 × HV:15.0 kV. X-ray diffraction (XRD) pattern was recorded by X-ray diffractometer (Germany, Bruker D8-advance, Cu Ka k = 1.54056 nm) for 2µ values over 10–700. The UV–Vis spectra were recorded on Shimadzu UV-1800 spectrophotometer at room temperature using quartz cell of 1 cm path length, multipoint Brunauere–Emmere–Teller (BET) method were used for the determination of specific surface area of the nanoparticle from the N2 adsorption data at the relative pressure (P/P0) range of 0.05–1.0, FT-IR spectrum has been taken using Brukers instrument and in the range of 4000–500 cm−1.
Reagents and materials
All the chemicals and reagents used in this work were of analytical grade and purchased from Merck (Merck, Darmstadt, Germany). The water used for aqueous phase was MiliQ water (A Grade) in every stage of the study.
Synthesis of ZnFeBONP
The ZnFeBONP were synthesized according to a co-precipitation method followed by magnetic stirring. First, 4.5 g of ZnCl2 and 7.4 g of FeCl3 were added to 300 ml of DW. Then, 35 ml of 1 M NaOH solution was added to the former solution dropwise. The whole solution is then stirred with magnetic stirrer for 2 h at 60 °C temperature, leading to smaller and more homogenized particles. A brown precipitate quickly formed, which was allowed to crystallize completely for another 30 min. The precipitate was washed with DW by magnetic decantation until the pH of the suspension was less than 7.5. The suspension was filtered by means of suction pump, after filtration precipitate was dried at about 100 °C and then crush into powder and use for further study.
Solution preparation and adsorption behavior
Adsorption behavior was performed by adding 0.05 g of ZnFeBONP to the 50 ml of CR solution in a 100 ml conical flask. The pH of the CR solution was adjusted at 6.5 using 0.01 molL−1 HCl or 0.1 mol L−1 NaOH and the solution was shaken on rotary shaker at 200 rpm for 90 min. The aliquot taken from the solution at a fixed time point was centrifuged at 2000 rpm for 3 min. The concentration of CR in the solution was measured spectrophotometrically at 498 nm. The concentration of CR decreased with time due to its adsorption by ZnFeBONP.
The adsorption capacity of each dye in the adsorption system, qe, was calculated by
where Ci and Ce are initial and equilibrium concentrations (mg L−1), respectively, m is the mass of the adsorbent (g); and V is volume of the solution (L).
Results and discussion
Characterization
The ZnFeBONP were synthesized according to a co-precipitation method at constant temp 60 °C with stirring. Figure S1a (Supplement file) shows typical SEM images of the ZnFeBONP. As shown in Fig. S1a, it is found that the product is composed of a large quantity of nanostructures. The low magnification image shows the particle size and the average size of nanoparticle was 37.72 nm. The TEM image of ZnFeBONP nanoparticles was shown in Fig. 1a, and the average particle size was about 20 nm, which is much smaller than the SEM morphology shown in Fig. 1a and it is good in terms of adsorption point of view. EDX was used to observe the elemental composition of the samples. The results reveal that the sample is composed of Zn, Fe and O in which the concentrations are about 21.76, 26.94 and 51.29 wt%, respectively (Fig. 1b). A FT-IR spectrum of ZnFeBONP is presented in Fig. 2. The absorption bands below 687 cm−1 is attributed to Fe–O stretching vibrational modes. The peak at 1631 cm−1 is ascribed to the O–H bending vibration of adsorbed water molecules and the broad absorption band at 3372 cm−1 corresponds to the O–H stretching region. The observed peak at 1351 cm−1 in the FT-IR spectrum of ZnFeBONP may be endorsed to C–O single bond. The band appeared at 1473 cm−1 may be due to C–H bending vibration. In Fig. 3, it could be seen that the curve belonged to the nitrogen sorption isotherm whose adsorption curve was not coincident with desorption curve thus forming a hysteresis loop. The calculated specific surface area was 133.3 m2 g−1. The X-ray diffraction patterns of ZnFeBONPs are shown in Fig. 4. Significant X-ray diffraction peaks occurred at 31.24°, 34.35°, 36.29°, 56.19°, 62.63° and 67.80° corresponds to reflection from (1 1 1), (2 0 0), (0 0 2), (1 0 2), (3 1 1) and (2 0 0) miller planes of ZnFeBONPs and the corresponding peaks became stronger and narrower, which implied that the crystallinity was higher. The structural characterization results obtained from the XRD pattern reveals that the powder is polycrystalline (cubic and hexagonal lattice).
Effect of pH solution
pH of solution has very deep impact on the adsorption process. In this study, the adsorption experiments have been conducted in the initial pH range from 5.0 to 8.5 and the corresponding result is shown in Fig. 5. The effect of the solution pH on the adsorption of dye onto ZnFeBONP was assessed at different values, ranging from 5 to 8.5. In these experiments, the initial concentration of dye and adsorbent dosage were set at 20 mg L−1 and 0.05 g, respectively, for all batch tests in this experiment. The % adsorption is maximum at pH 6.5 that is 98.88%. Therefore, further parameters were performed at pH 6.5. The surface of metal oxides is generally covered with hydroxyl groups that vary in form with pH. The experimentally calculated pHzpc of ZnFeBONPs is 7.1. The surface charge is positive at pH values lower than pHzpc, neutral at pHzpc, and negative at pH values higher than it. Therefore, adsorption efficiency decreases at pH value higher than 6.5.
Effect of time variation
The effect of contact time is a very crucial factor which persuades the adsorption of dye pollutant from wastewater. For this purpose, 50 mL of desired (20 mg L−1 CR dye) solution was prepared and further it was treated with 50 mg of adsorbent at 298 K, the CR were separated from aqueous solutions and the residual concentration of dye pollutant in aliquot were measured by spectrophotometric method. The results show a very high adsorption rate at initial times which is recognized to the high concentration of adsorption sites on the surface and strong interaction between the adsorbate and adsorbent (Fig. 6). Over time, the concentration gradient reduces due to the adsorption of CR molecules onto the adsorbent and leads to a lower adsorption during the later stages. The graph shows that within 20 min the 88% dye was adsorbed and at 40 min the adsorption reaches up to 92% and remains constant.
The effect of adsorbent concentration
The effect of ZnFeBONP quantity for % adsorption was investigated by adding various amounts of adsorbent in the range 0.05–0.2 g into the beaker containing 50 mL of dye solution (20 mg L−1) at pH 6.5 and 25 °C for all batch experiments. The results are shown in Fig. 7 and indicated that addition of 0.2 g of adsorbent per 50 mL solution of dye (20 mg L−1) lead to maximum adsorption of dye. The supernatant was analyzed for the remaining dye. The results showed that the adsorption efficiencies increased by increasing adsorbent dose. It was observed that by increasing the ZnFeBONP dosage from 0.05 to 0.2 g, the removal efficiency (%) of dye increased from 69.51 to 92.88%. This observation can be explained by the greater number of adsorption sites made available at greater ZnFeBONP dosages. Above 0.2 g of ZnFeBONP dosage, the adsorption equilibrium of dye was reached and the removal ratio of dye held almost no variety. Nevertheless, the adsorption capacity initially increased and then decreased as the ZnFeBONP dosage increased.
Chemical kinetic models (pseudo-second-order)
The pseudo-second-order model, proposed by Ho and McKay, can be represented in the following linear form [23].
In this model, plotting the t/qt of experimental data versus t is used to show its suitability for explanation of whole adsorption data. It was found that the plot of t/qt versus t is a straight line. The high and reasonable values of R2 and closeness of experimental and theoretical (qe) value indicate this model ability for explanation of adsorption process over the whole concentration range. Sorption kinetic studies were conducted at RT under initial dye concentration of 20 mg L−1. Adsorption kinetics were studied using kinetic model, namely the pseudo-second-order model.
The pseudo-second-order kinetic model is described by the formula
The results showed that the adsorption system followed the Ho and McKay equations for the entire adsorption. Kinetics of Congo red adsorption on ZnFeBONP best fit the pseudo-second-order model, suggesting that the adsorption mechanism depends on the adsorbate and adsorbent, and the rate-limiting step may be chemisorption involving valence forces through the sharing or exchange of electrons. This confirmed that adsorption of dye takes place probably via surface exchange reactions until the surface functional sites are fully occupied.
Adsorption isotherms
The equilibrium adsorption isotherm model, which is the number of mg adsorbed per gram of adsorbent (qe) vs. the equilibrium concentration of adsorbate (Fig. 9a), is fundamental in describing the interactive behavior between adsorbate and adsorbent. Analysis of isotherm data is important for predicting the adsorption capacity of the adsorbent, which is one of the main parameter required for the design of an adsorption system. Equilibrium isotherm studies were carried out with different initial concentrations of CR (50–200 mg L−1) at 25 °C and pH 6.0. Three models were used to analyze the equilibrium adsorption data: Langmuir, Freundlich and Temkin [24, 25]. Langmuir’s model does not take into account the variation in adsorption energy, but it is the simplest description of the adsorption process. It is based on the physical hypothesis that the maximum adsorption capacity consists of a monolayer adsorption, that there are no interactions between adsorbed molecules, and that the adsorption energy is distributed homogeneously over the entire coverage surface.
The general form of the Langmuir isotherm is:
where Ce is the equilibrium concentration of the CR in the solution (mg L−1), qe is the amount of CR adsorbed per unit mass of adsorbent (mg g−1), at equilibrium concentration, Ce, αL (L mg−1) and KL (Lg−1) are the Langmuir constants with αL related to the adsorption energy and qmax [= KL/αL] signifies the maximum adsorption capacity (mg g−1), which depends on the number of adsorption sites. The Langmuir isotherm shows that the amount of anions adsorbed increases as the concentration increases up to a saturation point. As long as there are available sites, adsorption will increase with increasing CR concentrations, but as soon as all of the sites are occupied, a further increase in concentrations of CR solutions does not increase the amount of CR on adsorbents (Table 1).
After linearization of the Langmuir isotherm, Eq. (3), we obtain
The values of αL and KL are calculated from the slope and intercept of the plot of Ce/qe vs. Ce (Fig. 8). The amount of CR adsorbed (mg g−1) was calculated based on a mass balance equation as given below:
where Co is the initial concentration of CR in mg L−1, V is the volume of experimental solution in L, and m is the dry weight of nanoparticles in g. The parameters of the Langmuir equation were calculated and are given in Table 2. Table 2 indicates that the maximum adsorption capacity of ZnFeBONP (qmax = 333.33 mg g−1) for CR is very much higher than that of other adsorbents.
The Freundlich equilibrium isotherm equation was also used to describe experimental adsorption data. This isotherm is an empirical equation which is used for the description of multilayer adsorption with interaction between adsorbed molecules. The Freundlich isotherm is the earliest known relationship describing the adsorption equation. The Freundlich empirical model is represented by
where Ce is the equilibrium concentration (mg L−1), qe is the amount adsorbed at equilibrium (mg g−1), and Kf (mg g−1) and 1/n are Freundlich constants depending on the temperature and the given adsorbent–adsorbate couple. n is related to the adsorption energy distribution, and Kf indicates the adsorption capacity. The linearized form of the Freundlich adsorption isotherm equation is
The values of Kf and 1/n calculated from the intercept and slope of the plot of ln qe vs. ln Ce (Fig. 9b) are listed in Table 2. Table 2 shows that the values of correlation coefficient R2 for the fit of experimental isotherm data to Langmuir equation is more close to 0.964 than that of Freundlich equation. Therefore, the Langmuir model represents the experimental data better on the basis of values of regression coefficients.
The Temkin isotherm contains a factor that explicitly accounts the interaction between adsorbing species–adsorbent. The Temkin equilibrium isotherm equation was also used to describe experimental adsorption data (Fig. 9c). The heat of the adsorption and the adsorbent–adsorbate interaction using Temkin isotherm model were evaluated [26].
where Ce is the equilibrium concentration (mg L−1), qe is the amount adsorbed at equilibrium (mgg−1), B is the Temkin constant related to heat of the adsorption (J mol−1), T is the absolute temperature (K), R is the universal gas constant (8.314 J mol−1 K−1), KT is the equilibrium binding constant (L mg−1). The values of the Temkin constants and the correlation coefficient are lower than the Langmuir value. Therefore, the Langmuir isotherm provides the best correlation for the experimental data. The correlation coefficients R2 obtained from Temkin model were comparable to that obtained for Langmuir and Freundlich equations, which explain the applicability of Temkin model to the adsorption of CR onto ZnFeBONP.
Separation factor
The essential feature of the Langmuir isotherm can be expressed in terms of a dimensionless constant separation factor (RL) given by the following equation:
RL values within the range 0 < RL < 1 indicate favorable adsorption (Fig. 9d) [27]. In this study, RL value of ZnFeBONP for the initial CR concentration of 50 mg L−1, obtained as 0.0476, indicate favorable adsorption of CR onto them. The Freundlich isotherm model is an empirical equation that describes the surface heterogeneity of the sorbent. It considers multilayer adsorption with a heterogeneous energetic distribution of active sites, accompanied by interactions between adsorbed molecules.
Adsorption thermodynamic parameters
Thermodynamic parameter related to the adsorption process i.e., free energy change (ΔG, kJ mol−1) for adsorption at 25 ◦C was calculated using the following equation:
where R is the universal gas constant (8.314 J mol−1 K−1), T is the temperature and KL is Langmuir constant (L mol−1) obtained from the plot of Ce/qe versus Ce. The calculated ΔG value was found to be − 2.430 kJ mol−1. The negative value of free energy change indicated the spontaneous nature of sorption and confirmed affinity of adsorbent for the dye [28, 29].
Regeneration of ZnFeBONP by heating
Regeneration capacity of an adsorbent shows the cost effectiveness of the adsorbent and this over-all process plays a key role in its commercial application. The regeneration was conducted by calcinations of used ZnFeBONP at 300 °C for 2 h. During calcinations, the adsorbed organic components were driven-off by its oxidative decomposition along with desorption of water molecules. Adsorption studies over regenerated ZnFeBONP were carried out at pre-optimized conditions. The process of regeneration and recycle of the adsorbent was carried out three times to confirm the performance in CR removal. The adsorption capacity after successive regeneration and use is presented in Fig. 10. After first regeneration, the ZnFeBONP exhibited about 5% lower adsorption capacities than the fresh sample. However, after second and third regenerations, the adsorption capacity remained constant. This suggests that, ZnFeBONP can be regenerated by calcination process with an acceptable adsorption capacity.
Industrial applications
The most important feature of any research is that it can be suitability applied for industry and environment. In the current work, synthesized ZnFeBONP absorbent is applied to dye removal of industrial waste samples. The variation in removal percentage, particularly at higher sample volume and concentration of dye molecule must not exceed ± 5% to suppose that the method can be applied as pilot plant. The efficiency of the present adsorbents for the removal of CR from real effluents was examined using two effluent streams. Thereafter, in our experiments, 50 mL of concentrated wastewater was diluted to 500 mL, and optimized procedure was carried out. The result shows the significant adsorption of CR take place, when the volume is large but again we carried out experiment at high volume and concentration. In all the examined approaches, using 0.2 g of adsorbent for 1 h, more than 98% of CR removal was achieved. All the experimental conditions, results and comparison data with other material are shown in [30–42] Tables 3 and 4, respectively. It was found that in most cases, more than 98% of dye removal was achieved. This confirms the usefulness of the adsorbent for the CR dye removal.
Conclusion
ZnFeBONP were synthesized according to a co-precipitation method at constant temp 60 °C with stirring an average size distribution of 37.72 nm and utilized it as an efficient adsorbent for removing of Congo red from wastewater samples. The effects of pH, contact time, adsorbent dosage, dye concentration on the removal of dye were investigated separately through batch experiments. Results indicated optimized pH 6.5, 40 min contact time, 0.2 mg adsorbent dose for 20 ppm dye. Isotherm modeling revealed that the Langmuir isotherm could better describe the adsorption of the dye onto the ZnFeBONP as compared to other models. Because of the high-specific surface area and nano-scale particle size, ZnFeBONP indicated favorable adsorption behavior for dye. The results showed that the adsorption system followed the Ho and McKay equations for the entire adsorption. Kinetics of Congo red adsorption on ZnFeBONp best fit the pseudo-second-order model. At the end of this study, we can say that the modified ZnFeBONP nanoparticles may be used as an alternative sorbent for the removal of some dye molecules from wastewaters.
Abbreviations
- α L :
-
Langmuir isotherm constant (L mg−1)
- C 0 :
-
Initial dye concentration in liquid phase (mg L−1)
- C e :
-
Liquid phase dye concentration at equilibrium (mg L−1)
- K s :
-
Equilibrium rate constant of pseudo-second-order adsorption (g mg−1 min−1)
- K f :
-
Freundlich constant (L g−1)
- K L :
-
Langmuir isotherm constant (L g−1)
- q e :
-
Amount of dye adsorbed at equilibrium (mg g−1)
- q t :
-
Amount of dye adsorbed at time t (mg g−1)
- Q max :
-
Maximum adsorption capacity of the adsorbent (mg g−1)
- m :
-
Mass of adsorbent used (g)
- n :
-
Freundlich isotherm exponent
- R 2 :
-
Linear correlation coefficient
- V :
-
Volume of dye solution (L)
- R L :
-
Separation factor
References
Ramalingam B, Khan MMR, Mondal B, Mandal AB, Das SK (2015) Facile synthesis of silver nanoparticles decorated magnetic-chitosan microsphere for efficient removal of dyes and microbial contaminants. ACS Sustain Chem Eng 3:2291–2302
Vîrlan C, Ciocârlan RG, Roman T, Gherca D, Cornei N, Pui A (2013) Studies on adsorption capacity of cationic dyes on several magnetic nanoparticles. Acta Chem IASI 21:19–30
Fisli A, Yusuf S, Ridwan YK, Krisnandi JG (2014) Preparation and characterization of magnetite-silica nano-composite as adsorbents for removal of methylene blue dyes from environmental water samples. Adv Mater Res 896:525–531
Jiang Z, Xie J, Jiang D, Yan Z, Jing J, Liu D (2014) Enhanced adsorption of hydroxyl contained/anionic dyes on nonfunctionalized Ni@SiO2core–shell nanoparticles: kinetic and thermodynamic profile. Appl Surf Sci 292:301–310
Haldorai Y, Shim J-J (2014) An efficient removal of methyl orange dye from aqueous solution byadsorption onto chitosan/MgO composite: a novel reusable adsorbent. Appl Surf Sci 292:447–453
Tang L, Cai Y, Yang G, Liu Y, Zeng G, Zhou Y, Li S, Wang J, Zhang S, Fang Y, He Y (2014) Cobalt nanoparticles-embedded magnetic ordered mesoporous carbon for highly effective adsorption of rhodamine B. Appl Surf Sci 314:746–753
Amaranatha Reddy D, Lee S, Choi J, Park S, Ma R, Yang H, Kim TK (2015) Green synthesis of AgI-reduced graphene oxide nanocomposites: toward enhanced visible-light photocatalytic activity for organic dye removal. Appl Surf Sci 341:175–184
Wang J, Tsuzuki T, Bin Tang A, Sun XJ, Dai GD, Rajmohan JL, Wang X (2014) Recyclable textiles functionalized with reduced graphene oxide@ZnO for removal of oil spills and dye pollutants. Aust J Chem 67:71–77
Kong LP, Gan XJ, bin Ahmad AL, Hamed BH, Evarts ER, Ooi BS, Lim JK (2012) Design and synthesis of magnetic nanoparticles augmented microcapsule with catalytic and magnetic bifunctionalities for dye removal. Chem Eng J 197:350–358
Deng J-H, Zhang X-R, Zeng G-M, Gong J-L, Niu Qiu-Ya, Liang J (2013) Simultaneous removal of Cd(II) and ionic dyes from aqueous solution using magnetic graphene oxide nanocomposite as an adsorbent. Chem Eng J 226:189–200
Zhang Y-R, Shen S-L, Wang S-Q, Huang J, Peng S, Wang Q-R, Zhao B-X (2014) A dual function magnetic nanomaterial modified with lysine for removal of organic dyes from water solution. Chem Eng J 239:250–256
Fegade U, Singh A, Krishna Chaitanya G, Singh N, Attarde S, Kuwar A (2014) Highly selective and sensitive receptor for Fe3+ probing. Spectrochim Acta Part A Mole Biomole Spectr 121:569–574
Fegade U, Tayade S, Krishna Chaitanya G, Attarde S, Kuwar A (2014) Fluorescent and chromogenic receptor bearing amine and hydroxyl functionality for iron(III) detection in aqueous solution. J Fluoresc 24:675–681
Fegade U, Saini A, Sahoo SK, Singh N, Bendre R, Kuwar A (2014) 2,2′-(Hydrazine-1,2-diylidenedimethylylidene) bis(6-isopropyl-3-methylphenol) based selective dual-channel chemosensor for Cu2+ in semiaqueous media. RSC Adv 4:39639–39644
Fegade U, Sahoo SK, Attarde S, Singh N, Kuwar A (2014) Colorimetric and fluorescent “On-Off” chemosensor for Cu2+ in semi-aqueous medium. Sens Actuators B 202:924–928
Fegade U, Sharma H, Attarde S, Singh N, Kuwar A (2014) Urea based dipodal fluorescence receptor for sensing of Fe3+ ion in semi-aqueous medium. J Fluoresc 24:27–37
Fegade U, Sharma H, Bondhopadhyay B, Basu A, Attarde S, Singh N, Kuwar A (2014) Turn-on” fluorescent dipodal chemosensor for nano-molar detection of Zn2+: application in living cells imaging. Talanta 125:418–424
Kuwar A, Fegade U, Tayade K, Patil U, Puschmann H, Gite V, Dalal D, Bendre RS (2013) Bis(2-hydroxy-3-isopropyl-6-methyl-benzaldehyde) ethylenediamine: a novel cationsensor. J Fluoresc 23:859–864
Fegade U, Attarde S, Sahoo SK, Singh N, Kuwar A (2014) A selective and discriminating noncyclic receptor for HSO4 − ion recognition: 2, 2-(pyridine-2,6-diylbis(azanediyl)) bis (methylene) diphennol. RSC Adv 4:15288–15292
Fegade U, Sahoo SK, Singh A, Singh N, Attarde S, Kuwar A (2015) Anal Chim Acta 872:63–69
Jethave G, Fegade U, Attarde S, Ingle S (2017) Facile synthesis of lead doped zinc-aluminum oxide nanoparticles (LD-ZAO-NPs) for efficient adsorption of anionic dye: kinetic, isotherm and thermodynamic behaviors. J Indus Eng Chem 53:294–306
Fegade U, Jethave G, Su K-Y, Huang W-R, Wu R-J (2018) An multifunction Zn0.3Mn0.4O4 nanospheres for carbon dioxide reduction to methane via photocatalysis and reused after fifth cycles for phosphate adsorption. J Environ Chem Eng. https://doi.org/10.1016/j.jece.2018.02.040
Crini G (2008) Kinetic and equilibrium studies on the removal of cationic dyes from aqueous solution by adsorption onto a cyclodextrin polymer. Dyes Pigments 415–426
Langmuir L (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Freundlich H, Heller W (1939) The adsorption of cis- and trans-azobenzene. J Am Chem Soc 61:2228–2230
Temkin MJ, Pyzhev V (1940) Acta Physiochim USSR 12:217–222
Weber TW, Chakravorti RK (1974) Pore and solid diffusion models for fixed-bed adsorbers. J Am Inst Chem Eng 209:228–238
Stumm W, Morgan JJ (1995) Aquatic chemistry: chemical equilibrium and rates in natural waters, 3rd edn. Wiley, New York, p 1040
Liu R, Hongxia F, Yin H, Lu PL, Tao Y (2015) A facile sol combustion and calcination process for the preparation of magnetic Ni0.5Zn0.5Fe2O4 nanopowders and their adsorption behaviors of Congo red. Powder Tech 274:418–425
Girgis E, Adel D, Tharwat C, Attallah O, Rao KV (2015) Cobalt ferrite nanotubes and porous nanorods for dye removal. Adv Nano Res 3:111–121
Yang S, Zhaohui W, Huang L, Zhou B, Lei M, Sun L, Tian Q, Pan J, Wei W, Zhang H (2014) Significantly enhanced dye removal performance of hollow tin oxide nanoparticles via carbon coating in dark environment and study of its mechanism. Nanoscale Res Lett 442:1–9
Liu R, Tian Y, Jintao X, Hongxia F, Li Y (2016) Efficient removal of Congo Red by magnetic CoFe2O4 nanoparticles prepared via the rapid combustion process. J Nanosci Nanotechnol 16:9535–9543
Liu R, Hongxia F, Yimin L, Yin H, Lequn Yu, Ma L, Han J (2016) Adsorption optimization and mechanism of neutral red onto magnetic Ni0.5Zn0.5Fe2O4/SiO2 nanocomposites. J Nanosci Nanotechnol 16:8252–8262
Liu R, Lv P, Hongxia F, Rongzhu L, Xiaoyang W, Yimin L (2017) Removal performance of methyl blue onto magnetic MgFe2O4 nanoparticles prepared via the rapid combustion process. J Nanosci Nanotechnol 17:4755–4762
Zheng Y, Zhu B, Chen H, You W, Jiang C, Jiaguo Yu (2017) Hierarchical flower-like nickel(II) oxide microspheres with high adsorption capacity of Congo red in water. J Colloid Interface Sci 504:688–696
Lei C, Zhu X, Zhu B, Yu J, Ho W (2016) Hierarchical NiO–SiO2 composite hollow microspheres with enhanced adsorption affinity towards Congo red in water. J Colloid Interface Sci. 466:238–246
Zhichao Yu, Liu B, Zhou H, Feng C, Wang X, Yuan K, Gan X, Zhu L, Zhang G, Dong X (2017) Mesoporous ZrO2 fibers with enhanced surface area and the application as recyclable absorbent. Appl Surf Sci 399:288–297
Xiaofei W, Wang W, Li F, Khaimanov S, Tsidaeva N, Lahoubi M (2016) PEG-assisted hydrothermal synthesis of CoFe2O4 nanoparticles with enhanced selective adsorption properties for different dyes. Appl Surf Sci 389:1003–1011
Lei C, Pi M, Jiang C, Cheng B, Jiaguo Yu (2017) Synthesis of hierarchical porous zinc oxide (ZnO) microspheres with highly efficient adsorption of Congo red. J Colloid Interface Sci 490:242–251
Aliabadi RS, Mahmoodi NO (2018) Synthesis and characterization of polypyrrole, polyaniline nanoparticles and their nanocomposite for removal of azo dyes; sunset yellow and Congo red. J Cleaner Prod 179:235–245
Ealias AM, Saravanakumar MP (2018) Facile synthesis and characterisation of AlNs using protein rich solution extracted from sewage sludge and its application for ultrasonic assisted dye adsorption: isotherms, kinetics, mechanism and RSM design. J Environ Manag 206:215–227
Xu J, Xu D, Zhu B, Cheng B, Jiang C (2018) Adsorptive removal of an anionic dye Congo red by flower-like hierarchical magnesium oxide (MgO)-graphene oxide composite microspheres. Appl Surf Sci 435:1136–1142
Acknowledgement
The authors are thankful to the Principal Prof. Dr. M. V. Waykole, President of TES Management Dr. M. M Phalak and Chairman of TES Management M. D. Phalak of the Bhusawal Arts, Science and P. O. Nahata Commerce College, Bhusawal, (MH), India, for providing necessary laboratory facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Jethave, G., Fegade, U. Design and synthesis of Zn0.3Fe0.45O3 nanoparticle for efficient removal of Congo red dye and its kinetic and isotherm investigation. Int J Ind Chem 9, 85–97 (2018). https://doi.org/10.1007/s40090-018-0140-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40090-018-0140-9