Abstract
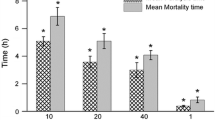
The use of traditional medicines has gained popularity with time. Bryonia laciniosa, Quercus infectoria, Putranjiva roxburghii and Mesua ferrea are used for treating several conditions as indigenous preparations (IPs). In view of potential harms caused by such preparations used during pregnancy, the authors attempted to investigate their safety using C. elegans as a host. Forty-eight IPs were collected; based on the presence of phenols and steroids, six were considered for the toxicity study in C. elegans. Survival assay analysis was performed for fixation of the dose (at 500 μg/ml) for the further conduct of the study. Disruption in events such as egg-laying capacity and progeny production was observed to predict the toxic effects of the compounds in IPs. Survival assay results at 30 and 70 h showed that survival of the worms varied according to the exposure. The worms exposed to IP extracts H1, H4, H9, F1, F4 and F9 showed 60%, 60%, 20%, 40%, 80% and 40% mortality at 70 h, respectively, compared to the vehicle control. A profound impact on reproductive toxicity was observed upon exposure to SSDs. The egg-laying capacity reduced to 19–57 compared to 101 in controls (P < 0.001) on Day 4. The progeny count reduced from 95 to 11–47 on Day 4 (P < 0.001). However, no gross morphological changes were observed. The present study revealed that IPs appeared to be toxic to the host system. However, corroboration of in vivo experiments in higher mammalian models is required for in-depth toxicology analyses and strengthening our evidence.






Similar content being viewed by others
References
WHO (2000) General guidelines for methodologies on research and evaluation of traditional medicine. World Health Organization, p 41
Byard RW et al (2017) What risks do herbal products pose to the Australian community? The Medical journal of Australia 206:86–90
Wachtel-Galor S, Benzie IFF (2011) Herbal medicine: An introduction to its history, usage, regulation, current trends, and research needs. In: Herbal medicine: biomolecular and clinical aspects
Neogi SB et al (2015) Indigenous medicine use for sex selection during pregnancy and risk of congenital malformations: a population-based case-control study in Haryana, India. Drug Saf 38:789–797
Manchanda S et al (2011) Sex ratio at birth in India, its relation to birth order, sex of previous children and use of indigenous medicine. PLoS ONE 6:e20097
Singh A, Sinha S (2016) Risk factors of congenital malformations in north india: a case control study. J Postgrad Med Edu Res 50:22–27
Bandyopadhyay S, Singh A (2003) History of son preference and sex selection in India and in the west. Bull Ind Inst Hist Med 33:149–167
Bandyopadhyay S, Singh A (2007) Sex Selection through traditional drugs in rural north India. Ind J Commun Med 32:32–34
Neogi S et al (2015) Consumption of indigenous medicines by pregnant women in North India for selecting sex of the foetus: what can it lead to? BMC Pregnancy Childbirth 15:208
Neogi SB et al (2016) Risk factors for stillbirth: findings from a population-based case-control study, Haryana, India. Paediatr Perinat Epidemiol 30:56–66
Ferretti P et al (2018) Mother-to-infant microbial transmission from different body sites shapes the developing infant gut microbiome. Cell Host Microbe 24:133–145.e135
Liang B et al (2015) Safety of Chinese herbal medicines during pregnancy. J Appl Toxicol 35:447–458
Nass R, Hamza I (2007) The nematode C. elegans as an animal model to explore toxicology in vivo: solid and axenic growth culture conditions and compound exposure parameters. Curr Protoc Toxicol 31:1–9
Tralau T et al (2012) Wind of change challenges toxicological regulators. Environ Health Perspect 120:1489–1494
Hartung T (2009) Toxicology for the twenty-first century. Nature 460:208–212
Knight AW et al (2009) Evaluation of high-throughput genotoxicity assays used in profiling the US EPA ToxCast chemicals. Regul Toxicol Pharmacol RTP 55:188–199
Olson H et al (2000) Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul Toxicol Pharmacol RTP 32:56–67
Hunt PR (2017) The C. elegans model in toxicity testing. J Appl Toxicol JAT 37:50–59
Leung MC et al (2008) Caenorhabditis elegans: an emerging model in biomedical and environmental toxicology. Toxicol Sci 106:5–28
Kaletta T, Hengartner MO (2006) Finding function in novel targets: C. elegans as a model organism. Nat Rev Drug Discov 5:387–398
Hope, I. (1999) Background on Caenorhabditis elegans. In: C. elegans a practical approach. Oxford: Oxford University Press
Hunt PR et al (2012) Toxicity ranking of heavy metals with screening method using adult C. elegans and propidium iodide replicates toxicity ranking in rat. Food Chem Toxicol 50:3280–3290
Harlow PH et al (2016) The nematode C. elegans as a tool to predict chemical activity on mammalian development and identify mechanisms influencing toxicological outcome. Sci Rep 6:22965
Kamaladevi A et al (2013) Lactobacillus casei protects malathion induced oxidative stress and macromolecular changes in C. elegans. Pestic Biochem Physiol 105:213–223
Stiernagle T (2006) Maintenance of C. elegans. WormBook [Internet]. Available from http://www.wormbook.org/chapters/www_strainmaintain/strainmaintain.html. Accessed 1 Feb 2018
Franke AA et al (1998) HPLC analysis of isoflavonoids and other phenolic agents from foods and from human fluids. Proc Soc Exp Biol Med 217:263–273
Shanker K, Gupta MM, Srivastava SK, Bawankule DU, Pal A, Khanuja SP (2007) Determination of bioactive nitrile glycoside(s) in drumstick (Moringa oleifera) by reverse phase HPLC. Food Chem 105:376–382
Murphy P et al (2009) Phytoestrogens in foods and plants: extraction and isolation march. J Med Food 2:107–109. https://doi.org/10.1089/jmf.1999.2.107
Kamaladevi A, Balamurugan K (2015) Role of PMK-1/p38 MAPK defense in C. elegans against Klebsiella pneumoniae infection during host-pathogen interaction. Pathog Dis 73:ftv021
Thompson G, de Pomerai DI (2005) Toxicity of short-chain alcohols to the nematode C. elegans: a comparison of endpoints. J Biochem Mol Toxicol 19:87–95
Katiki LM et al (2011) Caenorhabditis elegans as a model to screen plant extracts and compounds as natural anthelmintics for veterinary use. Vet Parasitol 182:264–268
Kamaladevi A, Balamurugan K (2014) Response of C. elegans during Klebsiella pneumoniae pathogenesis. BMC Infect Dis 14:P8–P8
Kesika P, Balamurugan K (2012) Studies on Shigella boydii infection in C. elegans and bioinformatics analysis of immune regulatory protein interactions. Biochimica et Biophysica Acta (BBA) Proteins Proteom 1824:1449–1456
Kamaladevi A et al (2016) Lactobacillus casei stimulates phase-II detoxification system and rescues malathion-induced physiological impairments in C. elegans. Comp Biochem Physiol Toxicol Pharmacol CBP 179:19–28
Zhang J-Q et al (2016) Chronic exposure to diquat causes reproductive toxicity in female mice. PLoS ONE 11:e0147075
Ganguli A et al (2018) Heavy metals in indigenous preparations used for sex selection during pregnancy in India. Biol Trace Element Res 188:239–244
Yu Z et al (2016) Multigenerational effects of heavy metals on feeding, growth, initial reproduction and antioxidants in C. elegans. PLoS ONE 11:e0154529
Tominaga N et al (2003) Caenorhabditis elegans responses to specific steroid hormones. J Health Sci 49:28–33
Chen P et al (2013) Metal-induced neurodegeneration in C. elegans. Frontiers in aging neuroscience 5:18–18
Scott CW et al (2013) Human induced pluripotent stem cells and their use in drug discovery for toxicity testing. Toxicol Lett 219:49–58
Candido EP, Jones D (1996) Transgenic C. elegans strains as biosensors. Trends Biotechnol 14:125–129
Prasanth MI et al (2016) Ultraviolet-A triggers photoaging in model nematode C. elegans in a DAF-16 dependent pathway. AGE 38:27
Prithika U et al (2016) External induction of heat shock stimulates the immune response and longevity of C. elegans towards pathogen exposure. Innate Immun 22:466–478
Marudhupandiyan S, Balamurugan K (2017) Intrinsic JNK-MAPK pathway involvement requires daf-16-mediated immune response during Shigella flexneri infection in C. elegans. Immunol Res 65:609–621
Dengg M, van Meel JC (2004) Caenorhabditis elegans as model system for rapid toxicity assessment of pharmaceutical compounds. J Pharmacol Toxicol Methods 50:209–214
Williams PL, Dusenbery DB (1988) Using the nematode C. elegans to predict mammalian acute lethality to metallic salts. Toxicol Ind Health 4:469–478
Boyd WA et al (2016) Developmental effects of the toxcast phase I and phase II chemicals in C. elegans and corresponding responses in zebrafish, rats, and rabbits. Environ Health Perspect 124:586–593
Meng F et al (2017) A Chinese herbal formula, Gengnianchun, Ameliorates β-amyloid peptide toxicity in a C. elegans model of Alzheimer’s disease. Evid Based Complement Altern Med 2017:10
Yu Y-B et al (2010) Cinnamomum cassia bark in two herbal formulas increases life span in C. elegans via insulin signaling and stress response pathways. PLoS ONE 5:e9339–e9339
Acknowledgements
The authors wish to sincerely thank the Department of Biotechnology, Alagappa University, Karaikudi, Tamil Nadu, for providing support in conducting the experiment in their campus laboratory. This study was conducted as part of a large study supported by the Department of Science and Technology, New Delhi, and Science and Technology Council, Haryana. The funding body had no role in the design of the study, collection, analysis and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors do not report any conflict of interest to publish this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Significant Statement
Traditional medicinal preparations are not always safe. Unregulated use of such preparations pronounces systematic investigation to ensure safety. Consumption of indigenous preparations for having a male child is reported from parts of India. Their intake during critical period of pregnancy (6–10 weeks), presence of phytoestrogens and steroids as constituents and its reported association with birth defects and stillbirths warrant thorough investigation through prospectively designed predictive toxicological studies. Not only this merits scientific attention, the social and health hazards embedded within this issue call for validation. Our study on exploring the toxicity of indigenous preparations strengthens this evidence for instituting corrective measures.
Rights and permissions
About this article
Cite this article
Rai, P., Rajasekharan, S., Ganguli, A. et al. Indigenous Preparations of Bryonia laciniosa, Quercus infectoria, Putranjiva roxburghii and Mesua ferrea Induce Developmental Toxicity in C. elegans. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 90, 657–667 (2020). https://doi.org/10.1007/s40011-019-01138-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-019-01138-1