Abstract
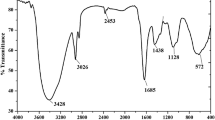
Immunological and biochemical properties of skin mucus of different fish species may have mutual beneficial effects on health management when the fishes are farmed together in same system. Present experiment was conducted to investigate and compare the immunological and biochemical properties of epidermal mucus of three brackishwater fishes, namely Lates calcarifer, Chanos chanos and Mugil cephalus. Mucus was collected from the dorso-lateral surface of six individual fish of each species and used for analysis of immunological properties and biochemical composition. Innate immune parameters such as lysozyme, haemolytic, phagocytic activities and lectin were significantly (p < 0.05) more prominent in the mucus of C. chanos followed by that of L. calcarifer and M. cephalus. Similarly, mucus of C. chanos exhibited maximum protease, alkaline phosphatase, and antibacterial activities. UV spectral analysis showed the presence of toluene, isoquinoline, 2-furaldehyde, octadecenoic acid, biphenyl, thymidine, and cinnamic acid in the mucus of L. calcarifer, whereas these were absent in other two species. Fourier transform-infrared spectrum analysis revealed that isothiocyanate, aldehyde and alkene were common functional groups in the mucus of all three fishes. The results indicated that mucus of C. chanos has stronger innate immune properties as compared to that of other two fishes and therefore, polyculture of this fish with other fish or shrimp species may have beneficial effects for disease prevention.









Similar content being viewed by others
References
Salinas I (2015) The mucosal immune system of teleost fish. Biology 4:525–539
Hellio C, Pons AM, Beaupoil C, Bourgougnon N, Gal YL (2002) Antibacterial, antifungal and cytotoxic activities of extracts from fish epidermis and epidermal mucus. Int J Antimicrob Agents 20(3):214–219
Bragadeeswaran S, Thangaraj S (2011) Haemolytic and antibacterial studies on skin mucus of eel fish, Anguilla linnaues 1758. Asian J Biol Sci 4(3):272–276
Cole AM, Weis P, Diamond G (1997) Isolation and characterization of pleurocidin, antimicrobial peptides in the skin secretions of winter flounder. J Biol Chem 272:12008–12013
Alexander JB, Ingram GA (1992) Noncellular nonspecific defence mechanisms of fish. Annu Rev Fish Dis 2:249–279
Ross NW, Firth KJ, Wang A, Burka JF, Johnson SC (2000) Changes in hydrolytic enzyme activities of naïve Atlantic salmon Salmo salar skin mucus due to infection with the salmon louse Lepeophtheirus salmonis and cortisol implantation. Dis Aquat Organ 41(1):43–51
Iger Y, Abraham M (1997) Rodlet cells in the epidermis of fish exposed to stressors. Tissue Cell 29(4):431–438
Eldani A, Primavera JH (1981) Effect of different stocking combinations on growth, production and survival of milkfish (Chanos chanos Forskal) and prawn (Penaeus monodon Fab.) in polyculture in brackishwater ponds. Aquaculture 23:59–72
Yuan D, Yi Y, Yakupitiyage A, Fitzimmons K, Diana JS (2010) Effects of addition of red tilapia (Oreochromis spp.) at different densities and sizes on production, water quality and nutrient recovery of intensive culture of white shrimp (Litopenaeus vannamei) in cement tanks. Aquaculture 298:226–238
Biswas G, Ananda Raja R, De D, Sundaray JK, Ghoshal TK, Anand S, Kumar S, Panigrahi A, Thirunavukkarasu AR, Ponniah AG (2012) Evaluation of productions and economic returns from two brackishwater polyculture systems in tide-fed ponds. J Appl Ichthyol 28:116–122
Aghuzbeni SHH, Hajirezaee Saeed, Matinfar Abbas, Khara Hossein, Ghobadi Mahmoud (2017) A preliminary study on polyculture of western white shrimp (Litopenaeus vannamei) with mullet (Mugil cephalus): an assessment of water quality, growth parameters, feed intake efficiency and survival. J Appl Anim Res 45(1):247–251
Monwar Md, Mostafa Ruhul Amin, Sarker AKM, Das Nani Gopal (2013) Polyculture of seabass with tilapia for the utilization of brown fields in the coastal areas of Cox’s Bazar. Bangladesh 5(6):104–109
Martinez-Porchas M, Martinez-Cordova LR, Porchas-Cornejo MA, EliaJA Lopez (2010) Shrimp polyculture: apotentially profitable, sustainable, but uncommon aquacultural practice. Rev Aquac 2:73–85
Cruz PS, Andalecio MN, Bolivar RB, Fitzsimmons K (2008) Tilapia–Shrimp polyculture in Negros Island, Philippines: a review. J World Aquac Soc 39:713–725
Priyadarsani L, Abraham TJ (2013) Ecology of antibiotic resistant vibrios in traditional shrimp farming system (bhery) of West Bengal, India. J Coast Life Med 1(4):265–272
Wibowo AM, Maftuch Fadjar (2015) Utilization of Tilapia Mucus to inhibit Vibrio harveyi on Vannamei (Litopenaeus vannamei). J Life Sci Biomed 5(5):141–148
Schultz DR, Perez N, Mendez AJ, Snodgrass D, Serafy JE, Prince ED, Crow WA, Capo TR (2007) Tracking gender factors in fish surface mucus: temporal patterns in individual koi (Cyprinus carpio). J Appl Ichthyol 23:184–188
Loganathan K, Arulprakash A, Prakash M, Senthilraja P (2013) Lysozyme, protease, alkaline phosphatase and esterase activity of epidermal skin mucus of freshwater snake head fish Channa striatus. Int J Res Pharm Biosci 3(1):17–20
Bauer AW, Kirby WMM, Sherris JC, Turck M (1996) Antibiotic susceptibility testing by a nstandardized single disc method. Am J Clin Pathol 45:493–496
Bing D, Weyand J, Satvitsky A (1967) Hemagglutination with aldehyde-fixed erythrocytes for assay of antigens and antibodies. Proc Soc Exp Biol Med 1244:1166–1170
Cupp-Enyard Carrie (2008) Sigma’s Non-specific protease activity assay—casein as a substrate. JOVE 18:889
Wel OY, Xavier R, Marimuthu K (2010) Screening of antibacterial activity of mucus extract of Snakehead fish, Channa striatus (Bloch). Eur Rev Med Pharmacol Sci 14:675–681
Jill P, Losey Zamzow GS (2002) Ultraviolet radiation absorbance by coral reef fish mucus: photo-protection and visual communication. Environ Biol Fishes 63:41–47
Douglas RH, Hawryshyn CW (1990) Behavioral studies of fish vision: an analysis of visual capabilities. In: Douglas RH, Djamgoz MBA (eds) The visual system of fish. Chapman and Hall, London, pp 373–418
Hellio C, Bremer G, Pons AM, Le Gal Y, Bour Gougnon N (2005) Inhibition of the development of microorganisms (bacteria and fungi) by extracts of marine algae from Brittany (France). Appl Microbiol Biotechnol 54:543–549
Noga M, Magarinos B, Toranzo AE, Lamas J (1995) Sequential pathology of experimental pasteurellosis in gilthead seabream Sparus aurata a light microscopic and electron microscopic study. Dis Aquat Organ 21:177–186
Matsushita M, Matsushita A, Endo Y, Nkata M, Kojima N, Fujita Mizuochi T (2004) Origin of the classical complement pathway: lamprey orthologue of mammalian C1q acts as a lectin. Proc Natl Acad Sci USA 101(27):10127–10131
Nigam AK, Kumari U, Mittal S, Mittal AK (2012) Comparative analysis of innate immune parameters of the skin mucous secretions from certain freshwater teleosts, inhabiting different ecological niches. Fish Physiol Biochem 38(5):1245–1256
Aranishi F, Mano N, Hirose H (1998) Fluorescence localization of epidermal cathepsins L and B in the Japanese eel. Fish Physiol Biochem 19(3):205–209
Cho JH, Park IY, Kim HS, Lee WT, Kim MS, Kim SC (2002) Cathepsin D produces antimicrobial peptide parasin I from histone H2A in the skin mucosa of fish. FASEB J 16(3):429–431
Acknowledgements
The authors are grateful to the Director, Central Institute of Brackishwater Aquaculture, for providing the necessary facilities to carry out this research work and the Indian Council of Agricultural Research, New Delhi, India, for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict among the authors regarding the research data presented in the paper.
Additional information
Significance statement
Finding of the study showed that the mucosal immunity of C. chanos is relatively stronger than that of L. calcarifer and M. cephalus, which will help in developing health management strategies in polyculture of milkfish with other fish or shrimp.
Rights and permissions
About this article
Cite this article
Kumar, P., Rajeshwaran, T., Priya, P. et al. Comparative Immunological and Biochemical Properties of the Epidermal Mucus from Three Brackishwater Fishes. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 89, 95–103 (2019). https://doi.org/10.1007/s40011-017-0923-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-017-0923-3