Abstract
The type-1 water soluble chlorophyll binding proteins (WSCP1) have been generally known as chlorophyll extractors and transporters from the thylakoid membrane to the chloroplast envelope, where the membrane bound chlorophyllase catabolizes the chlorophyll. Despite the type-2 WSCP, WSCP1 has been known to be located in the chloroplasts of the green plants. In the present study, the non-chloroplastic protein superfamily containing domain of unknown function 538 (DUF538) as functional homologue of type-1 WSCP has been identified in plants. The structural similarities/differences and the cellular locations of Celosia cristata DUF538 and Chenopodium album WSCP1 were predicted by using computational tools and the chlorophyll binding abilities of their purified maltose binding protein-fused forms were estimated by maltose binding affinity method. It was predicted that despite CaWSCP1, CcDUF538 is a non-chloroplastic protein. The chlorophyll binding abilities of the recombinant fusion forms of test WSCP1 and DUF538 were estimated to be about 58 and 56%, respectively. Considering DUF538 as stress-induced protein, it was speculated that they may form complex with chlorophyll molecules or their hydrolyzed products out of chloroplasts to proceed the chlorophyll breakdown and nitrogen/carbon recycling in stress-challenged plants.





Similar content being viewed by others
Abbreviations
- WSCP:
-
Water soluble chlorophyll binding proteins
- DUF:
-
Domain of unknown function
- BPI:
-
Bactericidal permeability increasing
- IPTG:
-
Isopropyl-1-thio-β-d-galactoside
- NBT:
-
Nitro blue tetrazolium
- BCIP:
-
5-bromo-1-chloro-3-indolyl phosphate
- DMF:
-
Dimethyl formamide
- Pchl:
-
Proto chlorophyll
- EDTA:
-
Ethylene diamine tetra acidic acid
- MBP:
-
Maltose-binding protein
References
Horigome D, Satoh H, Itoh N, Mitsunaga K, Oonishi I, Nakagawa A, Uchida A (2007) Structural mechanism and photoprotective function of water-soluble chlorophyll-binding protein. J Biol Chem 282:6525–6531
Noguchi T, Kamimura Y, Inoue Y, Itoh S (1999) Photoconversion of a water-soluble chlorophyll protein from Chenopodium album: resonance raman and fourier transform infrared study of protein and pigment structures. Plant Cell Physiol 40:305–310
Satoh H, Uchida A, Nakayama K, Okada M (2001) Water-soluble chlorophyll protein in Brassicaceae plants is a stress-induced chlorophyll-binding protein. Plant Cell Physiol 42:906–911
Takahashi S, Yoshikawa M, Kamada A, Ohtsuki T, Uchida A, Nakayama K, Satoh H (2013) The photoconvertible water-soluble chlorophyll-binding protein of Chenopodium album is a member of DUF538, a superfamily that distributes in Embryophyta. J Plant Physiol 170:1549–1552
Takahashi S, Aizawa K, Nakayama K, Satoh H (2015) Water soluble chlorophyll binding proteins from Arabidopsis thaliana and Raphanus sativus target the endoplasmic reticulum body. BMC Res Notes 8:365
Takahashi S, Yanai H, Oka-Takayama Y, Zanma-Sohtome A, Fujiyama K, Uchida A, Nakayama K, Satoh H (2013) Molecular cloning, characterization and analysis of the intracellular localization of a water-soluble chlorophyll binding protein (WSCP) from Virginia pepperweed (Lepidium virginicum), a unique WSCP that preferentially binds chlorophyll b in vitro. Planta 238:1065–1080
Yamada K, Hara-Nishimura I, Nishimura M (2011) Unique defense strategy by the endoplasmic reticulum body in plants. Plant Cell Physiol 52:2039–2049
Yamada K, Nagano AJ, Nishina M, Hara-Nishimura I, Nishimura M (2013) Identification of two novel endoplasmic reticulum body-specific integral membrane proteins. Plant Physiol 161:108–120
Gholizadeh A, BaghbanKohnehrouz B (2010) Identification of DUF538 cDNA clone from Celosia cristata expressed sequences of none stressed and stressed leaves. Russ J Plant Physiol 57:247–252
Gholizadeh A (2011) Heterologous expression of stress-responsive DUF538 domain containing protein and its morpho-biochemical consequences. Protein J 30:351–358
Gholizadeh A, Baghbankohnehrouz S (2013) DUF538 protein super family is predicted to be the potential homologue of bactericidal/permeability-increasing protein in plant system. Protein J 32:163–171
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Moran R (1982) Formulae for determination of chlorophyllous pigments extracted with N, N-dimethylformamide. Plant Physiol 69:1376–1381
Fang Z, Bouwkamp JC, Solomos T (1998) Chlorophyllase activities and chlorophyll degradation during leaf senescence in non-yellowing mutant and wild type of Phaseolus vulgaris L. J Exp Bot 49:503–510
Nakagami H, Sugiyama N, Mochida K, Daudi A, Yoshida Y, Toyoda T, Tomita M, Ishihama Y, Shirasu K (2010) Large-scale comparative phosphoproteomics identifies conserved phosphorylation sites in plants. Plant Physiol 153:1161–1174
Annamalai P, Yanagihara S (1999) Identification and characterization of a heat-stress induced gene in cabbage encodes a kunitz type protease inhibitor. J Plant Physiol 155:226–233
Downing WL, Mauxion F, Fauvarque MO, Reviron MP, de Vienne D, Vartanian N, Giraudat J (1992) A Brassica napus transcript encoding a protein related to the Künitz protease inhibitor family accumulates upon water stress in leaves, not in seeds. Plant J 2:685–693
Nishio N, Satoh H (1997) A water-soluble chlorophyll protein in cauliflower may be identical to BnD22, a drought-induced, 22-kilodalton protein in rapeseed. Plant Physiol 115:841–846
Satoh H, Nakayama K, Okada M (1998) Molecular cloning and functional expression of a water-soluble chlorophyll protein, a putative carrier of chlorophyll molecules in cauliflower. J Biol Chem 273:30568–30575
Takamiya KI, Tsuchiya T, Ohta H (2000) Degradation pathway (s) of chlorophyll: what has gene cloning revealed? Trends Plant Sci 5:426–431
Tsuchiya T, Ohta H, Masuda T, Mikami B, Kita N, Shioi Y, Takamiya K (1997) Purification and characterization of two isozymes of chlorophyllase from mature leaves of Chenopodium album. Plant Cell Physiol 38:1026–1031
Schenk N, Schelbert S, Kanwischer M, Goldschmidt EE, Dörmann P, Hörtensteiner S (2007) The chlorophyllases AtCLH1 and AtCLH2 are not essential for senescence-related chlorophyll breakdown in Arabidopsis thaliana. FEBS Lett 27:5517–5525
Takahashi S, Yanai H, Nakamaru Y, Uchida A, Nakayama K, Satoh H (2012) Molecular cloning, characterization and analysis of the intracellular localization of a water-soluble chlorophyll-binding protein from Brussels sprouts (Brassica oleracea var. gemmifera). Plant Cell Physiol 53:879–891
Acknowledgements
The author of this paper is thankful to the Research Institute for Fundamental Sciences (RIFS), University of Tabriz for the financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest in this study.
Appendix
Appendix

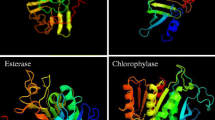
Prediction and comparison of primary, secondary and tertiary structures of WSCP1 and DUF538 proteins
Rights and permissions
About this article
Cite this article
Gholizadeh, A. Chlorophyll Binding Ability of Non-chloroplastic DUF538 Protein Superfamily in Plants. Proc. Natl. Acad. Sci., India, Sect. B Biol. Sci. 88, 967–976 (2018). https://doi.org/10.1007/s40011-016-0834-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40011-016-0834-8