Abstract
Purpose
The purpose of this study to establish the safety and efficacy data of Bojungikgi-tang (BJIGT) soft extract by determining the clinical pharmacokinetics (PKs) of its main ingredients and their active metabolites after oral administration.
Methods
A randomized, open-label, single-dose, single-center study on 12 healthy Korean male subjects was conducted. The plasma concentration of the active ingredients in BJIGT soft extract was determined in UPLC-MS/MS. Phoenix WinNonlin (version 8.1, Pharsight®, a Certara™ Company, Princeton, NJ, USA) was used to conduct compartmental and non-compartmental (NCA) analyses to assess PK parameters.
Results
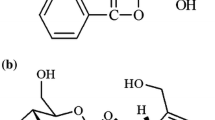
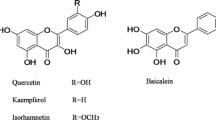
The PK parameters of ginsenoside Rb1 (Rb1) and glycyrrhizin (GL) were well described with 1-compartment analysis without lag time, and the 1-compartment model with combined transit compartment model and first-order absorption was used to evaluate the parameters of glycyrrhetinic acid (GLA). PK parameters of Rb1, GL and GLA including the clearance (CL/F), the volume of distribution (V/F), the rate of absorption (Ka), the maximum concentration (Cmax), time to reach maximum concentration (Tmax), the area under the curve of a plasma concentration versus time profile (AUC0-inf), and the elimination half-life (T1/2) were successfully estimated.
Conclusion
This is the first report to evaluate the PKs of major active ingredients and their metabolites after oral administration of BJIGT soft extract to Korean subjects.



Similar content being viewed by others
References
Alolga RN, Fan Y, Zhang G, Li J, Zhao YJ, Lelu Kakila J, Qi LW (2015) Pharmacokinetics of a multicomponent herbal preparation in healthy Chinese and African volunteers. Sci Rep 5:12961. https://doi.org/10.1038/srep12961
Bhagwat S, Haytowitz DB (2015) USDA database for the flavonoid content of selected foods. Release 3.2. Nutrient Data Laboratory. Beltsville Human Nutrition Research Center. Agricultural Research Service. U.S. Department of Agriculture. Retrieved from https://data.nal.usda.gov/system/files/Flav3.2.pdf
Byeon JC, Ahn JB, Jang WS, Lee S-E, Choi J-S, Park J-S (2018) Recent formulation approaches to oral delivery of herbal medicines. J Pharm Investig 49(1):17–26. https://doi.org/10.1007/s40005-018-0394-4
Choi M-K, Jin S, Jeon J-H, Kang WY, Seong SJ, Yoon Y-R, Song I-S (2018) Tolerability and pharmacokinetics of ginsenosides Rb1, Rb2, Rc, Rd, and compound K after single or multiple administration of red ginseng extract in human beings. J Ginseng Res. https://doi.org/10.1016/j.jgr.2018.10.006
Erlund I, Silaste ML, Alfthan G, Rantala M, Kesaniemi YA, Aro A (2002) Plasma concentrations of the flavonoids hesperetin, naringenin and quercetin in human subjects following their habitual diets, and diets high or low in fruit and vegetables. Eur J Clin Nutr 56(9):891–898. https://doi.org/10.1038/sj.ejcn.1601409
FDA (2018) The guidance for industry: bioanalytical method validation. Center for Drug Evaluation and Research. Retrieved from https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bioanalytical-method-validation-guidance-industry
He M, Chen W, Wang M, Wu Y, Zeng J, Zhang Z, Jiang J (2017) Simultaneous determination of multiple bioactive components of Bu-zhong-yi-qi-tang in rat tissues by LC-MS/MS: application to a tissue distribution study. J Chromatogr B Analyt Technol Biomed Life Sci 1044–1045:177–184. https://doi.org/10.1016/j.jchromb.2017.01.023
Hu L, Yao Z, Qin Z, Liu L, Song X, Dai Y, Yao X (2019) In vivo metabolic profiles of Bu-Zhong-Yi-Qi-Tang, a famous traditional Chinese medicine prescription, in rats by ultra-high-performance liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry. J Pharm Biomed Anal 171:81–98. https://doi.org/10.1016/j.jpba.2019.04.001
Hye-Sun Lim YJK, Sohn E, Yoon J, Kim B-Y, Jeong S-J (2018) Bojungikgi-Tang, a traditional herbal formula, exerts neuroprotective effects and ameliorates memory impairments in alzheimer's disease-like experimental models. Nutrients 10(12):1952. https://doi.org/10.3390/nu10121952
Hyun D-S, Kim J-D, Park J-H, Park S-J, Song C-H, Ku S-K (2015) Effects of Bojungikkitang (a Polyherbal Formula), on Gefitinib Pharmacokinetics in Rats. International Journal of Pharmacology 11(6):604–610. https://doi.org/10.3923/ijp.2015.604.610
Jeong JS, Ryu BH, Kim JS, Park JW, Choi WC, Yoon SW (2010) Bojungikki-tang for cancer-related fatigue: a pilot randomized clinical trial. Integr Cancer Ther 9(4):331–338. https://doi.org/10.1177/1534735410383170
Kanaze FI, Bounartzi MI, Georgarakis M, Niopas I (2007) Pharmacokinetics of the citrus flavanone aglycones hesperetin and naringenin after single oral administration in human subjects. Eur J Clin Nutr 61(4):472–477. https://doi.org/10.1038/sj.ejcn.1602543
Kim D-H, Jang I-S, Lee H-K, Jung E-A, Lee K-Y (1996) Metabolism of glycyrrhizin and baicalin by human intestinal bacteria. Arch Pharm Res 19(4):292–296. https://doi.org/10.1007/bf02976243
Kim HK (2013) Pharmacokinetics of ginsenoside Rb1 and its metabolite compound K after oral administration of Korean Red Ginseng extract. J Ginseng Res 37(4):451–456. https://doi.org/10.5142/jgr.2013.37.451
Kočevar Glavač N, Kreft S (2012) Excretion profile of glycyrrhizin metabolite in human urine. Food Chem 131(1):305–308. https://doi.org/10.1016/j.foodchem.2011.08.081
Lee JA, Jang S, Jun JH, Lee MS, Lee E, Kim N, Lee DH (2018) Herbal medicine (Bojungikki-tang) for allergic rhinitis: A protocol for a systematic review of controlled trials. Medicine (Baltimore) 97(3):e9551. https://doi.org/10.1097/MD.0000000000009551
Lee MY, Shin IS, Jeon WY, Seo CS, Ha H, Huh JI, Shin HK (2012) Protective effect of Bojungikki-tang, a traditional herbal formula, against alcohol-induced gastric injury in rats. J Ethnopharmacol 142(2):346–353. https://doi.org/10.1016/j.jep.2012.04.043
Michael I, Cohen LMG, OlgaBlumenfeld O, Irwin Arias M (1969) Gamma Glutamyl Transpeptidase: Measurement and Development in Guinea Pig Small Intestine. Pediatr Res 3(1):5–10. https://doi.org/10.1203/00006450-196901000-00001
Ministry of Food and Drug Safety. (2019).Korean Herbal Pharmacopoeia. (2019–72). Retrieved from https://www.mfds.go.kr/index.do
Mori K (1999) Effect of Hochu-ekki-to (TJ-41), a Japanese herbal medicine, on the survival of mice infected with influenza virus. Antiviral Res 44:103–111
Silveira JQ, Cesar TB, Manthey JA, Baldwin EA, Bai J, Raithore S (2014) Pharmacokinetics of flavanone glycosides after ingestion of single doses of fresh-squeezed orange juice versus commercially processed orange juice in healthy humans. J Agric Food Chem 62(52):12576–12584. https://doi.org/10.1021/jf5038163
Suzuki T, Tsukahara M, Akasaka Y, Inoue H (2017) A highly sensitive LC-MS/MS method for simultaneous determination of glycyrrhizin and its active metabolite glycyrrhetinic acid Application to a human pharmacokinetic study after oral administration. Biomed Chromatogr. https://doi.org/10.1002/bmc.4032
Yamamura Y, Kawakami J, Santa T, Kotaki H, Uchino K, Sawada Y, Iga T (1992) Pharmacokinetic profile of glycyrrhizin in healthy volunteers by a new high-performance liquid chromatographic method. J Pharm Sci 81(10):1042–1046. https://doi.org/10.1002/jps.2600811018
Yoo JH, Yim SV, Lee BC (2018) Study of Pharmacodynamic and Pharmacokinetic Interaction of Bojungikki-Tang with Aspirin in Healthy Subjects and Ischemic Stroke Patients. Evid Based Complement Alternat Med 2018:9727240. https://doi.org/10.1155/2018/9727240
Acknowledgement
This work was supported by a grant of the National Development Institute of Korean Medicine funded by the Korean Ministry of Health and Welfare, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
All authors declare that they have no conflict of interest.
Statement of human and animal rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional Review Board of Oriental Medicine Hospital of Wonkwang University (WKIRB approval number:2017–09) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was confirmed by the Institutional Review Board of Oriental Medicine Hospital of Wonkwang University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Choi, EJ., Choi, GW., Kim, J.H. et al. Pharmacokinetics of bioactive components after oral administration of Bojungikgi-tang in Korean subjects. J. Pharm. Investig. 50, 593–602 (2020). https://doi.org/10.1007/s40005-020-00488-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-020-00488-7