Abstract
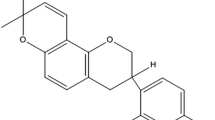
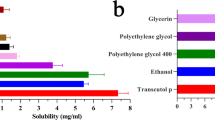
Vesicular carriers (VCs) offer enhanced and sustained delivery of drugs. The aim of this study was to explore the effects of ethanol concentration in VCs on topical delivery of a poorly water-soluble drug, using griseofulvin as prototype. VCs containing varying quantities of ethanol were prepared by solvent evaporation using Phospholipon® 90H (P90H) and characterized for entrapment efficiency (EE), morphology, size and size distribution, stability, viscosity and skin retention. Permeation profiles were assessed using rat skin and Franz diffusion cell and withdrawn samples analyzed spectrophotometrically. Spherical vesicles of average size of 137.70 ± 51.62 nm and polydipersity index of 0.555 were produced. Vesicle sizes decreased with increase in ethanol concentration. EE of 68.0 ± 5.6% was obtained for the optimized formulation. Differential scanning calorimetry indicated reversible perturbation of the skin layers as the mechanism of permeation. Permeation generally increased with increase in ethanol concentration. Ethosomal nanovesicular carriers encapsulating griseofulvin were formulated, which showed potentials for sustained and enhanced delivery through rat skin in direct proportion with ethanol concentration.





Similar content being viewed by others
References
Agubata CO, Nzekwe IT, Attama AA, Müller-Goymann CC, Onunkwo GC (2015) Formulation, characterization and anti-malarial activity of homolipid-based artemether microparticles. Int J Pharm 478:202–222
Attama AA, Reichl S, Müller-Goymann CC (2008) Diclofenac sodium delivery to the eye: in vitro evaluation of novel solid lipid nanoparticle formulation using human cornea construct. Int J Pharm 355:307–313
Attama AA, Reichl S, Müller-Goymann CC (2009) Sustained release and permeation of timolol from surface-modified solid-lipid nanoparticles through bioengineered human cornea. Curr Eye Res 34:698–705
Aulton ME (1999) Pharmaceutics: the science of dosage form design. Ist edn. Churchill Livingstone, Edinburgh
Barry BW (2001) Novel mechanisms and devices to enable successful transdermal drug delivery. Eur J Pharm Sci 14(2):101–114
Bendas ER, Tadros MI (2007) Enhanced transdermal delivery of salbutamol sulfate via ethosomes. AAPS Pharm Sci Tech 8(4):E107
Brookfield Engineering Labs Inc (2014) More solutions to sticky problems: a guide to getting MORE from your Brookfield Viscometers 1–50. http://www.brookfieldengineering.com/education/viscosity_whymeasure.asp. Accessed 28 Feb 2014
Carter SJ (1987) Jellies formulation: sodium alginate. In: Cooper and gun’s dispensing for pharmaceutical students, 12th edn. Churchill Livingstone, Edinburgh, pp 214–218
Chernysheva YV, Babak VG, Kildeeva NR, Boury F, Benoit JP, Ubrich N, Maincent P (2003) Effect of the type of hydrophobic polymers on the size of nanoparticles obtained by emulsion-solvent evaporation. Mendeleev Commun 13:65–68
Chu KA, Yalkowsky SH (2009) An interesting relationship between drug absorption and melting point. Int J Pharm 373:24–40
Cilurzo F, Albert E, Minghetti P, Gennari CGM, Casiraghi A, Montanari L (2010) Effect of drug chirality on the skin permeability of ibuprofen. Int J Pharm 386:71–76
El Khyat A, Mavon A, Leduc M, Agache P, Humbert P (1996) Skin critical surface tension. Skin Res Technol 2(2):91–96
El Maghraby GM, Barry BW, Williams AC (2008) Liposomes and skin: from drug delivery to model membranes. Eur J Pharm Sci 34:203–222
Elsayed MMA, Abdallah OY, Naggar VF, Khalafallah NM (2007) Lipid vesicles for skin delivery of drugs: reviewing three decades of research. Int J Pharm 332:1–16
Ghafourian T, Samaras EG, Brooks JD, Riviere JE (2010) Validated models for predicting skin penetration from different vehicles. Eur J Pharm Sci 41:612–616
Goodman M, Barry BW (1989) Action of penetration enhancers on human stratum corneum as assessed by differential scanning calorimetry. In: Bronaugh RL, Maibach HI (eds) Percutaneous absorption, 2nd edn. Marcel Dekker, New York, pp 567–595
GuhaSarkar S, Banerjee R (2010) Intravesical drug delivery: challenges, current status, opportunities and novel strategies. J Control Release 148:147–159
Jain S, Tiwary AK, Sapra B, Jain NK (2007) Formulation and evaluation of ethosomes for transdermal delivery of lamivudine. AAPS Pharm Sci Tech 8(4):Article111. https://doi.org/10.1208/pt0804111
Karavelidis V, Giliopoulos D, Karavas E, Bikiaris DN (2010) Nanoencapsulation of a water soluble drug in biocompatible polyesters: effects of polyesters melting point and glass transition temperature on drug release behavior. Eur J Pharm Sci 41:636–643
Kulkarni PV, Roney CA, Antich PP, Bonte FJ, Raghu AV, Aminabhavi TM (2010) Quinoline-n-butylcyanoacrylatebased nanoparticles for brain targeting for the diagnosis of Alzheimer’s disease. WIREs Nanomed Nanobiotechnol 2:35–47
Kumar S, Malick AW, Meltzer NM, Mouskountankis JD, Behl CR (1992) Studies of in-vitro skin permeation and retention of a leucotriene antagonist from topical vehicles with a hairless guinea pig model. J Pharm Sci 81:631–634
Lee PJ, Langer R, Shastri VP (2005) Role of n-methyl pyrrolidone in the enhancement of aqueous phase transdermal transport. J Pharm Sci 94:912–917
Lipoid GmbH (2017) Phospholipids. www.lipoid.com/en/phospholipids. Accessed 3 Nov 2017
Lopez-Pinto JM, Gonzalez-Rodriguez ML, Rabasco AM (2005) Effect of cholesterol and ethanol on dermal delivery from DPPC liposomes. Int J Pharm 298:1–12
Mandawgade SD, Patravale VB (2008) Development of SLNs from natural lipids: application to topical delivery of tretinoin. Int J Pharm 363:132–138
Martin A, Swarbrick J, Cammarata A (1983) Physical pharmacy, 3rd edn. Lea and Febiger, Philadelphia, pp 445–468
Marto J, Vitor C, Guerreiro A, Severino C, Eleutério C, Ascenso A, Simões S (2016) Ethosomes for enhanced skin delivery of griseofulvin. Colloids Surf B 146:616–623
Mbah CC, Builders PF, Attama AA (2014a) Nanovesicular carriers as alternative drug delivery systems: ethosomes in focus. Exp Opin Drug Deliv 11(1):45–59
Mbah C, Builders P, Nzekwe I, Kunle O, Adikwu M, Attama A (2014b) Formulation and in vitro evaluation of pH-responsive ethosomes for vaginal delivery of metronidazole. J Drug Deliv Sci Technol 24(6):565–571
Merck Index (1989) Stearic, oleic and linoleic acids, 11th edn. 1107, 1192, 1319. Merck & Co., Inc., New Jersey
Nguyen CA, Konan-Kouakou YN, Allemann E, Doelker E, Quintanar-Guerrero D, Fessi H, Gurny R (2006) Preparation of surfactant-free nanoparticles of methacrylic acid copolymers used for film coating. AAPS Pharm Sci Tech 7(3):63. http://www.aapspharmscitech.org
Saito Y, Sato T, Anazawa I (1989) Correlation between distribution of oxyethylene chains of nonionic surfactants and stability of cyclohexane droplets. Colloid Surf 40:107–114
Schreier H, Bouwstra J (1994) Liposomes and niosomes as topical drug carriers: dermal and transdermal drug delivery. J Control Release 30:1–15
Semete-Makokotlela B, Kalombo L, Hayeshi R, Swai H (2011) Nanomedicine for improved efficacy of tuberculosis drugs. 4th ANDI Stakeholders Meeting 24–28 Oct., Addis Ababa
Shakeel F, Baboota S, Ahuja A, Ali J, Shafiq S (2008) Skin permeation mechanism and bioavailability enhancement of celecoxib from transdermally applied nanoemulsion. J Nanobiotechnol 6:8. https://doi.org/10.1186/1477-3155-6-8
Shivanand P, Manish G, Viral D, Jarina F (2009) Transferosomes: a novel approach for transdermal drug delivery. Der Pharm Lett 1(2):143–150. http://scholarsresearchlibrary.com/archive.html
Takano R, Sugano K, Higashida A, Hayashi Y, Machida M, Aso Y, Yamashita S (2006) Oral absorption of poorly water-soluble drugs: computer simulation of fraction absorbed in humans from a mini-scale dissolution test. Pharm Res 23:1144–1156
Touitou E (1996) Composition of applying active substances to or through the skin. US Patent, 5,716,638
Touitou E, Dayan N, Bergelson L, Godin B, Eliaz M (2000) Ethosomes – novel vesicular carriers for enhanced delivery: characterization and skin penetration properties. J Control Release 65:403–418
United States Pharmacopoeia (USP) 26 (2003) Asian ed., United States Pharmacopoeial Convention, Inc, Pharmacopoeia Rockville, p 875
Williams A (2003) Transdermal and topical drug delivery, 1st edn. Pharmaceutical Press, London
Xiong GL, Quan D, Maibach HI (1996) Effects of penetration enhacers on in vitro percutaneous absorption of low molecular weight heparin through human skin. J Control Release 42:289–296
Acknowledgements
We thank NIPRD, Abuja and Sheda Science and Technology Complex (SHESTCO), Abuja, Nigeria for some of the facilities utilized in this research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Declaration of conflict of interest
The authors declare no conflict of interest in this research work. No sponsorship was received in carrying out this work and while preparing the article.
Research involving human and animal participants
No human subjects were used for this study. All the institutional and national guidelines for the care and use of laboratory animals were followed in accordance with the ethical procedures of NIPRD, Abuja, Nigeria (Number: 05:3:06), in line with the National Institute of Health guidelines, as revised, 1985, for handling of laboratory animals.
Rights and permissions
About this article
Cite this article
Mbah, C.C., Builders, P.F., Agubata, C.O. et al. Development of ethosomal vesicular carrier for topical application of griseofulvin: effect of ethanol concentration. J. Pharm. Investig. 49, 27–36 (2019). https://doi.org/10.1007/s40005-017-0367-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-017-0367-z