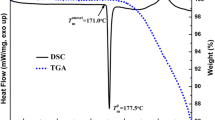
Abstract
New blends of hydroxypropylmethylcellulose (HPMC, 4000 cps) and Gelucire®44/14 (GE) were utilized to modulate the solubility and release rate of poorly water-soluble drug in a controlled manner. HPMC was used as sustained release polymer while GE was blended as a solubilizing carrier. The binary blends of HPMC and GE with proportional ratios (0, 25, 50, 70, 100%) were prepared by three different preparation methods: simple physical mixing, solvent evaporation and hot-melting. The physical properties such as surface morphology, thermal behavior and crystallinity pattern of the binary blends without loading drugs were then characterized using scanning electron microscopy (SEM), differential scanning calorimetry (DSC), and powder X-ray diffraction (PXRD), respectively. Finally, the ternary solid dispersions (SD) were prepared by dispersing model drugs in a binary blend. Two model drugs, water-soluble acetaminophen (AAP) and poorly water-soluble pranlukast (PLK) were applied to the binary blends. In case of AAP, HPMC retarded release rate but GE had no significant solubilizing effect due to the high AAP solubility, In contrast, the release rate of PLK was efficiently modulated release rate in a controlled manner with an aid of HPMC and GE. Surely, GE could play a key role in enhancing the dissolution rate while HPMC efficiently controlled release rate of drugs without losing drug crystallinity.





Similar content being viewed by others
References
Acevedo A, Takhistov P, de la Rosa CP, Florián V (2014) Thermal gelation of aqueous hydroxypropylmethylcellulose solutions with SDS and hydrophobic drug particles. Carbohydr Polym 102:74–79
Brough C, Williams RO 3rd (2013) Amorphous solid dispersions and nano-crystal technologies for poorly water-soluble drug delivery. Int J Pharm 453:157–166
Cao QR, Choi YW, Cui JH, Lee B-J (2005) Formulation, release characteristics and bioavailability of novel monolithic hydroxypropylmethylcellulose matrix tablets containing acetaminophen. J Control Release 108:351–361
Cao QR, Choi JS, Liu Y, Xu WJ, Yang M, Lee B-J, Cui JH (2013) A formulation approach for development of HPMC-based sustained release tablets for tolterodine tartrate with a low release variation. Drug Dev Ind Pharm 39:1720–1730
Chono S, Takeda E, Seki T, Morimoto K (2008) Enhancement of the dissolution rate and gastrointestinal absorption of pranlukast as a model poorly water-soluble drug by grinding with gelatin. Int J Pharm 347:71–78
da Fonseca Antunes AB, De Geest BG, Vervaet C, Remon JP (2013) Gelucire 44/14 based immediate release formulations for poorly water-soluble drugs. Drug Dev Ind Pharm 39:791–798
De Robertis S, Bonferoni MC, Elviri L, Sandri G, Caramella C, Bettini R (2015) Advances in oral controlled drug delivery: the role of drug-polymer and interpolymer non-covalent interactions. Expert Opin Drug Deliv 12:441–453
El-Badry M, Fetih G, Fathy M (2009) Improvement of solubility and dissolution rate of indomethacin by solid dispersions in Gelucire 50/13 and PEG4000. Saudi Pharm J 17:217–225
Faisal W, Ruane-O’Hora T, O’Driscoll CM, Griffin BT (2013) A novel lipid-based solid dispersion for enhancing oral bioavailability of Lycopene–In vivo evaluation using a pig model. Int J Pharm 453:307–314
Fernandez S, Chevrier S, Ritter N, Mahler B, Demarne F, Carrière F, Jannin V (2009) In vitro gastrointestinal lipolysis of four formulations of piroxicam and cinnarizine with the self emulsifying excipients Labrasol® and Gelucire® 44/14. Pharm res 26:1901–1910
Grohganz H, Priemel PA, Lobmann K, Nielsen LH, Laitinen R, Mullertz A, Van den Mooter G, Rades T (2014) Refining stability and dissolution rate of amorphous drug formulations. Expert Opin Drug Deliv 11:977–989
Grund J, Koerber M, Walther M, Bodmeier R (2014) The effect of polymer properties on direct compression and drug release from water-insoluble controlled release matrix tablets. Int J Pharm 469:94–101
Huang Y, Dai WG (2014) Fundamental aspects of solid dispersion technology for poorly soluble drugs. Acta Pharm Sin B 4:18–25
Jain AK, Soderlind E, Viriden A, Schug B, Abrahamsson B, Knopke C, Tajarobi F, Blume H, Anschutz M, Welinder A, Richardson S, Nagel S, Abrahmsen-Alami S, Weitschies W (2014) The influence of hydroxypropyl methylcellulose (HPMC) molecular weight, concentration and effect of food on in vivo erosion behavior of HPMC matrix tablets. J Control Release 187:50–58
Jang SW, Choi YW, Kang MJ (2014) Preparation of solid dispersion of Everolimus in Gelucire 50/13 using melt granulation technique for enhanced drug release. Bull Korean Chem Soc 35:1939
Jannin V (2009) Lauroyl polyoxylglycerides, functionalized coconut oil, enhancing the bioavailability of poorly soluble active substances. Ol Corps Gras Lipides 16:267–272
Kalantzi L, Reppas C, Dressman JB, Amidon GL, Junginger HE, Midha KK, Shah VP, Stavchansky SA, Barends DM (2006) Biowaiver monographs for immediate release solid oral dosage forms: acetaminophen (paracetamol). J Pharm Sci 95:4–14
Laitinen R, Lobmann K, Strachan CJ, Grohganz H, Rades T (2013) Emerging trends in the stabilization of amorphous drugs. Int J Pharm 453:65–79
Meena A, Parikh T, Gupta SS, Serajuddin AT (2014) Investigation of thermal and viscoelastic properties of polymers relevant to hot melt extrusion, II: cellulosic polymers. J Excip Food Chem 5:46–55
Mistry P, Mohapatra S, Gopinath T, Vogt FG, Suryanarayanan R (2015) Role of the strength of drug-polymer interactions on the molecular mobility and crystallization inhibition in ketoconazole solid dispersions. Mol Pharm 12:3339–3350
Park JB, Lim J, Kang CY, Lee B-J (2013) Drug release-modulating mechanism of hydrophilic hydroxypropylmethylcellulose matrix tablets: distribution of atoms and carrier and texture analysis. Curr Drug Deliv 10:732–741
Park JB, Park YJ, Kang CY, Lee B-J (2015) Modulation of microenvironmental pH and utilization of alkalizers in crystalline solid dispersion for enhanced solubility and stability of clarithromicin. Arch Pharm Res 38:839–848
Paudel A, Worku ZA, Meeus J, Guns S, Van den Mooter G (2013) Manufacturing of solid dispersions of poorly water soluble drugs by spray drying: formulation and process considerations. Int J Pharm 453:253–284
Piao Z-Z, Choe J-S, Oh KT, Rhee Y-S, Lee B-J (2014) Formulation and in vivo human bioavailability of dissolving tablets containing a self-nanoemulsifying itraconazole solid dispersion without precipitation in simulated gastrointestinal fluid. Eur J Pharm Sci 51:67–74
Sarode AL, Wang P, Obara S, Worthen DR (2014) Supersaturation, nucleation, and crystal growth during single- and biphasic dissolution of amorphous solid dispersions: polymer effects and implications for oral bioavailability enhancement of poorly water soluble drugs. Eur J Pharm Biopharm 86:351–360
Shaw LR, Irwin WJ, Grattan TJ, Conway BR (2005) The effect of selected water-soluble excipients on the dissolution of paracetamol and Ibuprofen. Drug Dev Ind Pharm 31:515–525
Shimpi SL, Chauhan B, Mahadik K, Paradkar A (2005) Stabilization and improved in vivo performance of amorphous etoricoxib using Gelucire 50/13. Pharm Res 22:1727–1734
Sutananta W, Craig DQ, Newton JM (1994) An investigation into the effect of preparation conditions on the structure and mechanical properties of pharmaceutical glyceride bases. Int J Pharm 110:75–91
Svensson A, Neves C, Cabane B (2004) Hydration of an amphiphilic excipient, Gelucire® 44/14. Int J Pharm 281:107–118
Tran TT-D, Tran PH-L (2013) Investigation of polyethylene oxide-based prolonged release solid dispersion containing isradipine. J Drug Deliv Sci Technol 23:269–274
Tran T-H, Park C, Kang T, Park Y-J, Oh E, Lee B-J (2015) Micromeritic properties and instrumental analysis of physical mixtures and solid dispersions with adsorbent containing losartan: comparison of dissolution-differentiating factors. Powder Technol 272:269–275
Van Nguyen H, Park C, Oh E, Lee B-J (2016a) Improving the dissolution rate of a poorly water-soluble drug via adsorption onto pharmaceutical diluents. J Drug Deliv Sci Technol 35:146–154
Van Nguyen H, Nguyen VH, Lee B-J (2016b) Dual release and molecular mechanism of bilayered aceclofenac tablet using polymer mixture. Int J Pharm 515:233–244
Vo CL, Park C, Lee B-J (2013) Current trends and future perspectives of solid dispersions containing poorly water-soluble drugs. Eur J Pharm Biopharm 85:799–813
Wang L, Hao Y, Liu N, Ma M, Yin Z, Zhang X (2012) Enhance the dissolution rate and oral bioavailability of pranlukast by preparing nanosuspensions with high-pressure homogenizing method. Drug Dev Ind Pharm 38:1381–1389
Acknowledgements
This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation funded by the Ministry of Science, ICT & Future Planning (2013M3A9B5075841), The Republic of Korea. The authors declare that there is no conflict of interest regarding the publication of this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Kyung-Ho Lee and Chulhun Park have equally contributed to this study.
Rights and permissions
About this article
Cite this article
Lee, KH., Park, C., Oh, G. et al. New blends of hydroxypropylmethylcellulose and Gelucire 44/14: physical property and controlled release of drugs with different solubility. J. Pharm. Investig. 48, 313–321 (2018). https://doi.org/10.1007/s40005-017-0322-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40005-017-0322-z