Abstract
Purpose
In infections of the Central Nervous System (iCNS), rapid identification of causing pathogens is crucial for survival and to avoid long-term sequelae. Targeted therapy may reduce side effects and development of antibiotic resistance. New molecular-based syndromic tests such as the “meningitis/encephalitis panel” (MEP) allow accelerated pathogen identification from cerebrospinal fluid. We conducted a clinical study to evaluate the MEP’s efficacy in paediatric patients.
Methods
Cohort study in a unique clinical setting by comparing the outcome data of two neighbouring Children’s Hospitals in Germany which are comparable in size, catchment area and equipment but differ regarding availability of the MEP: study centre 1 (SC1): yes; SC2: no. The study population included 213 paediatric patients with a suspected iCNS (SC1: 106; SC2: 107), with comparable age, CRP at admission and frequency of intensive care. The primary outcome was total use of antibiotics.
Results
Total antibiotic use per patient was numerically lower in SC1 than in SC2 (SC1: median 2.83 days; SC2 3.67 days; p = 0.671). Multiple linear regression analysis did not show a relevant association between MEP-availability and total antibiotic use (ß = 0.1, 95% confidence interval [−1.46; +1.67], p = 0.897). In the subcohort with suspected meningoencephalitis (SC1: 18, SC2: 17), duration of acyclovir treatment was shorter in SC1 than in SC2 (median 1.3 days vs. 2.7 days, descriptive p = 0.0397).
Conclusions
The add-on use of the MEP in paediatric patients with suspected iCNS was associated with a non-significant reduction in total antibiotic use, and with a reduced exposure to acyclovir in treated patients.

Similar content being viewed by others
Data availability
The original data of this study are available with the authors upon reasonable request.
Code availability
The codes used for data analysis in RStudio are available with the authors upon reasonable request.
Notes
Both hospitals are located in the central cities of their respective governmental districts, Middle Palatinate and Upper Palatinate, and are comparable in size, equipment and catchment area. The population sizes of both cities Weiden and Amberg are nearly identical: Weiden, 42,494; Amberg, 42,348 16. Eurostat, Demografische Indikatoren: Bevölkerung. Weiden in der Oberpfalz, Kreisfreie Stand; Amberg, Kreisfreie Stadt. [https://www.google.com/publicdata/explore?ds=mo4pjipima872_&met_y=population&idim=subregion3:DE233:DE231&hl=de&dl=de#!ctype=l&strail=false&bcs=d&nselm=h&met_y=population&scale_y=lin&ind_y=false&rdim=country_group&idim=subregion3:DE233:DE231&ifdim=country_group&hl=de&dl=de&ind=false]. The two hospitals are Academic Teaching Hospitals of the University Hospitals located nearest (SC1: Regensburg, SC2: Regensburg and Erlangen-Nurnberg).
The first primary endpoint was the total amount of all types of antiinfectives compared between the study sites, the other endpoints were the three different subclasses of antiinfective treatment (in the order antiviral, antibacterial, antifungal treatment).
Direct cost for the Biofire® FilmArray 2.0 system: 42,000 EUR (acquired in 2018, incl. taxes), for L2 service contract 3,500 EUR annually, and cost for each panel per patient is 145 EUR.
The MEP was approved by the FDA in 2015. Its key characteristics are simultaneous testing of a variety of pathogens from small sample volumes (200 µl CSF), rapid turnaround time (around one hour), and an easy-to-use system requiring few minutes hands-on time by lab personnel. Biomérieux Diagnostics: BIOFIRE® FILMARRAY® ME Panel [https://www.biomerieux-diagnostics.com/filmarray-meningitis-encephalitis-me-panel]. Pathogens detected by the MEP: 6 bacteria (Escherichia coli K1, Haemophilus influenzae, Listeria monocytogenes, Neisseria meningitidis, Streptococcus agalactiae, Streptococcus pneumoniae); 7 viruses (Cytomegalovirus (CMV); Enterovirus, Herpes Simplex virus type 1 (HSV-1), Herpes Simplex virus type 2 (HSV-2), Human Herpes virus 6 (HHV-6), Human Parechovirus (HPeV), Varicella zoster virus (VZV)) and one yeast (Cryptococcus neoformans/gattii).
The patient had normal inflammatory parameters and WBC count in the CSF. Following negative CSF cultures, antibiotic therapy was discontinued after 5 days, and he was discharged in good general state.
We used the following predictors: age (in days), sex, general state, CRP at admission, WBC count in CSF, outcome of CSF cultures, outcome of blood cultures, outcome of urine cultures, presence of a facial palsy, seizure prior to or at admission, typical symptoms of meningitis.
We used the following predictors: age (in days), sex, general state, CRP at admission, WBC count in CSF, outcome of CSF cultures, outcome of blood cultures, outcome of urine cultures, presence of a facial palsy, seizure prior to or at admission, typical symptoms of meningitis, results of cerebral imaging, EEG results and admission to ICU/NICU.
Patient was discharged against explicit medical advice (ID 32).
Two cases received targeted antibiotic therapy following detection of streptococcus pneumoniae in the MEP.
Abbreviations
- (i)CNS:
-
(Infections of the) Central Nervous System
- CSF:
-
Cerebrospinal fluid
- GVIF:
-
Generalized variance inflation factor
- HPeV:
-
Human Parechovirus
- IQR:
-
Interquartile range
- MEP:
-
Multiplex PCR meningitis/encephalitis panel
- SC:
-
Study center
- TBEV:
-
Tick-borne encephalitis virus
- WBC:
-
White blood cell
References
Global Burden of Disease Paediatrics C, Kyu HH, Pinho C, Wagner JA, Brown JC, Bertozzi-Villa A, Charlson FJ, Coffeng LE, Dandona L, Erskine HE et al: Global and National Burden of Diseases and Injuries Among Children and Adolescents Between 1990 and 2013: Findings From the Global Burden of Disease 2013 Study. JAMA Pediatr 2016, 170(3):267–287.
DGPI Handbuch- Infektionen bei Kindern: Meningitis, 6 edn: Georg Thieme Verlag KG; 2013, pp 727–729.
Chandran A, Herbert H, Misurski D, Santosham M. Long-term sequelae of childhood bacterial meningitis: an underappreciated problem. Pediatr Infect Dis J. 2011;30(1):3–6.
Brouwer MC, Tunkel AR, van de Beek D. Epidemiology, diagnosis, and antimicrobial treatment of acute bacterial meningitis. Clin Microbiol Rev. 2010;23(3):467–92.
Nigrovic LE, Malley R, Macias CG, Kanegaye JT, Moro-Sutherland DM, Schremmer RD, Schwab SH, Agrawal D, Mansour KM, Bennett JE, et al. Effect of antibiotic pretreatment on cerebrospinal fluid profiles of children with bacterial meningitis. Paediatrics. 2008;122(4):726–30.
Leazer R, Erickson N, Paulson J, Zipkin R, Stemmle M, Schroeder AR, Bendel-Stenzel M, Fine BR: Epidemiology of Cerebrospinal Fluid Cultures and Time to Detection in Term Infants. Paediatrics. 2017, 139(5).
Gerber JS, Ross RK, Bryan M, Localio AR, Szymczak JE, Wasserman R, Barkman D, Odeniyi F, Conaboy K, Bell L, et al. Association of broad- vs narrow-spectrum antibiotics with treatment failure, adverse events, and quality of life in children with acute respiratory tract infections. JAMA. 2017;318(23):2325–36.
Lindbaek M. Broad-spectrum antibiotics gave no clinical benefit and more adverse effects than narrow-spectrum antibiotics in treating acute respiratory tract infections in US children. BMJ Evid Based Med. 2019;24(3):119–20.
Website: World Health Organization / Fact sheets / Detail / Antimicrobial resistance. World Health Organization (WHO); accessed October 9th, 2020.
Biomérieux Diagnostics: Biofire Film Array Systems [https://www.biomerieux-diagnostics.com/filmarrayr-multiplex-pcr-system]
Biomérieux Diagnostics: BIOFIRE® FILMARRAY® ME Panel [https://www.biomerieux-diagnostics.com/filmarray-meningitis-encephalitis-me-panel]
Boudet A, Pantel A, Carles MJ, Bocle H, Charachon S, Enault C, Stephan R, Cadot L, Lavigne JP, Marchandin H. A review of a 13-month period of FilmArray Meningitis/Encephalitis panel implementation as a first-line diagnosis tool at a university hospital. PLoS ONE. 2019;14(10): e0223887.
Leber AL, Everhart K, Balada-Llasat JM, Cullison J, Daly J, Holt S, Lephart P, Salimnia H, Schreckenberger PC, DesJarlais S, et al. Multicenter evaluation of BioFire FilmArray meningitis/encephalitis panel for detection of bacteria, viruses, and yeast in cerebrospinal fluid specimens. J Clin Microbiol. 2016;54(9):2251–61.
Naccache SN, Lustestica M, Fahit M, Mestas J, Dien Bard J one year in the life of a rapid syndromic panel for meningitis/encephalitis: a paediatric tertiary care facility's experience. J Clin Microbiol. 2018, 56(5).
Arora HS, Asmar BI, Salimnia H, Agarwal P, Chawla S, Abdel-Haq N. Enhanced identification of group B streptococcus and escherichia coli in young infants with meningitis using the biofire filmarray meningitis/encephalitis panel. Pediatr Infect Dis J. 2017;36(7):685–7.
Eurostat, Demografische Indikatoren: Bevölkerung. Weiden in der Oberpfalz, Kreisfreie Stadt; Amberg, Kreisfreie Stadt. [https://www.google.com/publicdata/explore?ds=mo4pjipima872_&met_y=population&idim=subregion3:DE233:DE231&hl=de&dl=de#!ctype=l&strail=false&bcs=d&nselm=h&met_y=population&scale_y=lin&ind_y=false&rdim=country_group&idim=subregion3:DE233:DE231&ifdim=country_group&hl=de&dl=de&ind=false]
Gordis L. Epidemiology. 3rd ed. Philadelphia, Pennsylvania: Elsevier; 2004.
Krebs R: Costs for BIOFIRE Multiplex PCR System. Phone conversation and E-Mail with S.C. Disse, February 4th, 2022.
DGPI Handbuch- Infektionen bei Kindern: Antimikrobielle Chemotherapie, 6 edn: Georg Thieme Verlag KG; 2013.
Referenzperzentile für anthropometrische Maßzahlen und Blutdruck aus der Studie zur Gesundheit von Kindern und Jugendlichen in Deutschland (KiGGS). In: Gesundheitsberichterstattung des Bundes- GBE. 2th edn: Robert-Koch Institute; 2013: 26–32.
Graf EH, Farquharson MV, Cardenas AM. Comparative evaluation of the FilmArray meningitis/encephalitis molecular panel in a paediatric population. Diagn Microbiol Infect Dis. 2017;87(1):92–4.
Nabower AM, Miller S, Biewen B, Lyden E, Goodrich N, Miller A, Gollehon N, Skar G, Snowden J. Association of the FilmArray meningitis/encephalitis panel with clinical management. Hosp Pediatr. 2019;9(10):763–9.
Evans M, Merkel KG, Harder J, Rose DT Impact of the implementation of a rapid meningitis/encephalitis multiplex polymerase chain reaction panel on IV acyclovir duration: multicenter, retrospective cohort of adult and paediatric patients. Diagn Microbiol Infect Dis. 2019:114935.
O’Brien MP, Francis JR, Marr IM, Baird RW. Impact of cerebrospinal fluid multiplex assay on diagnosis and outcomes of central nervous system infections in children: a before and after cohort study. Pediatr Infect Dis J. 2018;37(9):868–71.
Posnakoglou L, Siahanidou T, Syriopoulou V, Michos A: Impact of cerebrospinal fluid syndromic testing in the management of children with suspected central nervous system infection. Eur J Clin Microbiol Infect Dis. 2020.
Hagen A, Eichinger A, Meyer-Buehn M, Schober T, Huebner J. Comparison of antibiotic and acyclovir usage before and after the implementation of an on-site FilmArray meningitis/encephalitis panel in an academic tertiary paediatric hospital: a retrospective observational study. BMC Pediatr. 2020;20(1):56.
Peros T, van Schuppen J, Bohte A, Hodiamont C, Aronica E, de Haan T: Neonatal bacterial meningitis versus ventriculitis: a cohort-based overview of clinical characteristics, microbiology and imaging. Eur J Pediatr. 2020.
Dack K, Pankow S, Ablah E, Zackula R, Assi M. Contribution of the BioFire((R)) FilmArray((R)) Meningitis/Encephalitis Panel: Assessing Antimicrobial Duration and Length of Stay. Kans J Med. 2019;12(1):1–3.
Lee SH, Chen SY, Chien JY, Lee TF, Chen JM, Hsueh PR. Usefulness of the FilmArray meningitis/encephalitis (M/E) panel for the diagnosis of infectious meningitis and encephalitis in Taiwan. J Microbiol Immunol Infect. 2019;52(5):760–8.
Wendt S, Trawinski H, von Braun A, Lubbert C. Durch Zecken ubertragbare Erkrankungen: Von der Lyme-Borreliose über das Q-Fieber bis zur FSME. CME (Berl). 2019;16(5):53–71.
Crook J, Xu M, Slaughter JC, Willis J, Browning W, Estrada C, Gay J, Thomas G, Benton A, Quinn C, et al. Impact of clinical guidance and rapid molecular pathogen detection on evaluation and outcomes of febrile or hypothermic infants. Infect Control Hosp Epidemiol. 2020;41(11):1285–91.
Eichinger A, Hagen A, Meyer-Buhn M, Huebner J. Clinical benefits of introducing real-time multiplex PCR for cerebrospinal fluid as routine diagnostic at a tertiary care paediatric center. Infection. 2019;47(1):51–8.
Fachinformation- Aciclovir Hospira 25 mg/ml, Konzentrat zur Herstellung einer Infusionslösung. Pfizer; 2016. http://fachinformation.srz.de/pdf/pfizerpharma/aciclovirhospira25mgmlpfizer.pdf.
Rao S, Abzug MJ, Carosone-Link P, Peterson T, Child J, Siparksy G, Soranno D, Cadnapaphornchai MA, Simoes EA: Intravenous acyclovir and renal dysfunction in children: a matched case control study. J Pediatr 2015, 166(6):1462–1468 e1461–1464.
Downes KJ, Boge CLK, Baro E, Wharton GT, Liston KM, Haltzman BL, Emerson HM, Doe E, Fulchiero R, Tran V, et al. Acute kidney injury during treatment with intravenous acyclovir for suspected or confirmed neonatal herpes simplex virus infection. J Pediatr. 2020;219(126–132): e122.
Sen SS, Sil A, Chakraborty U, Chandra A: Stevens-Johnson syndrome-toxic epidermal necrolysis: a fatal cutaneous adverse reaction to oral acyclovir. BMJ Case Rep 2020, 13(8).
Piccirilli G, Chiereghin A, Gabrielli L, Giannella M, Squarzoni D, Turello G, Felici S, Vocale C, Zuntini R, Gibertoni D, et al. Infectious meningitis/encephalitis: evaluation of a rapid and fully automated multiplex PCR in the microbiological diagnostic workup. New Microbiol. 2018;41(2):118–25.
Radmard S, Reid S, Ciryam P, Boubour A, Ho N, Zucker J, Sayre D, Greendyke WG, Miko BA, Pereira MR, et al. Clinical Utilization of the FilmArray Meningitis/Encephalitis (ME) Multiplex Polymerase Chain Reaction (PCR) Assay. Front Neurol. 2019;10:281.
Park SE, Lim TJ, Nam SO, Chang CL, Byun SY, Ko A, Kong J, Cho JW, Yeon GM, Lee YJ: Clinical utility of the FilmArray meningitis/encephalitis panel in children at a tertiary center in South Korea. Brain Dev 2020.
Agut H, Bonnafous P, Gautheret-Dejean A. Laboratory and clinical aspects of human herpesvirus 6 infections. Clin Microbiol Rev. 2015;28(2):313–35.
Olijve L, Jennings L, Walls T: Human Parechovirus: an Increasingly Recognized Cause of Sepsis-Like Illness in Young Infants. Clin Microbiol Rev. 2018, 31(1).
Britton PN, Dale RC, Nissen MD, Crawford N, Elliott E, Macartney K, Khandaker G, Booy R, Jones CA, Investigators P-A. Parechovirus encephalitis and neurodevelopmental outcomes. Paediatrics. 2016;137(2): e20152848.
Weichelt B, Hooper E, Chow B. Infant identical triplets’ presentation of human parechovirus Type 3. IDCases. 2019;15: e00494.
Britton PN, Walker K, McMullan B, Galea C, Burrell R, Morgan B, Honan I, Teutsch S, Smithers-Sheedy H, Fairbairn N, et al. Early life parechovirus infection neurodevelopmental outcomes at 3 years: a cohort study. J Pediatr. 2020;219(111–117): e111.
Anand V, Holmen J, Neely M, Pannaraj PS, Dien Bard J. The brief case: neonatal meningitis caused by listeria monocytogenes diagnosed by multiplex molecular panel. J Clin Microbiol. 2016;54(12):2846–9.
Richards RJ, Simon MS, Phillips CD, Lief L, Jenkins SG, Satlin MS: Rapid detection of Listeria monocytogenes rhombencephalitis in an immunocompetent patient by multiplexed PCR. BMJ Case Rep 2018, 2018.
Soucek DK, Dumkow LE, VanLangen KM, Jameson AP. Cost justification of the BioFire FilmArray meningitis/encephalitis panel versus standard of care for diagnosing meningitis in a community hospital. J Pharm Pract. 2019;32(1):36–40.
Duff S, Hasbun R, Ginocchio CC, Balada-Llasat JM, Zimmer L, Bozzette SA. Economic analysis of rapid multiplex polymerase chain reaction testing for meningitis/encephalitis in paediatric patients. Future Microbiol. 2018;13:617–29.
Messacar K, Robinson CC, Dominguez SR. Letter to the editor: economic analysis lacks external validity to support universal syndromic testing for suspected meningitis/encephalitis. Future Microbiol. 2018;13:1553–4.
Kassambara A: Regression Model Diagnostics: Multicollinearity Essentials and VIF in R. WebSource. 2018. http://www.sthda.com/english/articles/39-regression-model-diagnostics/160-multicollinearity-essentials-and-vif-in-r/. Accessed 1 June 2020.
Acknowledgements
We thank Ms. Claudia Kick, personal secretary to the Head of Children’s Hospital Weiden, for assistance with data entry in Children’s Hospital Weiden.
Funding
All authors received no funding for the conduct of the study or for the writing of this manuscript.
Author information
Authors and Affiliations
Contributions
AM had the original study idea and initiated the study. SD planned the study in detail, collected the data, did the statistical analysis and drafted the manuscript. AZ supervised the study conduct and data analysis and critically revised the manuscript. AM supervised the laboratory investigations. AF, FS and AM helped in logistic issues of the study and critically revised the manuscript. HH assisted with data collection and critically revised the manuscript. SD conducted the study as her postgraduate master’s thesis in epidemiology at Mainz University/ Germany.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicting interests to disclose.
Ethics approval
The responsible Ethics Committee approved the study (Bavarian Chamber of Physicians, Correspondence No. 2019-140). Formal consent by individual patients was waived because this study used only fully pseudonymized datasets.
Consent to participate
Consent by individual patients was waived by the responsible Ethics Committee because we retrospectively analyzed fully pseudonymized datasets.
Consent for publication
All authors approved the final version of the manuscript and consent for publication.
Supplementary Information
Below is the link to the electronic supplementary material.
Appendix
Appendix
Patient identification and data collection
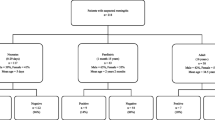
We firstly identified all patients who had received a lumbar puncture as coded by the German procedure classification “OPS” (German: “Operationen- und Prozedurenschluessel” meaning surgery and procedure key) as OPS 1-204.2: “lumbar puncture” electronically from the hospital records. After reviewing all individual patients’ medical records, we manually excluded all patients who had received a lumbar puncture for other reasons than a suspected iCNS, i.e. elective neurometabolic diagnostics in refractory epilepsy or suspected idiopathic intracranial hypertension, and we excluded all cases in which no/insufficient CSF specimen for pathogen detection could be obtained. Lumbar puncture due to a malignancy was not performed in any patient, either. The following flow chart documents the selection process and the final study population (Fig. 2). Data for most variables were extracted manually from patients’ electronic charts. Some data including diagnosis-related groups and results from CSF cultures were assessed in an automated manner, using the hospitals’ electronic databases. We carefully checked all data for accuracy, internal consistency and completeness. All datasets were reviewed at least twice.
Study Flow Chart showing identification of patients with suspected meningitis/ encephalitis from the pool of patients who received a lumbar puncture in 2017 or in 2018.The numbers in brackets equal the respective number of patients in 2017 (first number) and from 2018 (second number), respectively. All patients were inpatients in either SC1 or SC2
Statistical methods/ statistical analysis plan
Comparability of the study sites was descriptively analysed using pre-defined baseline characteristics (age, gender, CRP, body temperature and general condition at admission). The general state at admission was categorized into one of three groups according to the clinical status as assessed by the paediatrician in charge (“good”/ “very good” / “excellent” = 1; “stable” / “fair” = 2; “poor”/ “severely reduced” or admitted directly to ICU = 3). The main analysis comprised comparisons of the two study sites SC1 and SC2 (i.e., MEP available vs. MEP non-available). Numeric covariates were described as appropriate for their distributions (mean and standard deviation for normal distributed variables, median and quartiles otherwise). Because of the skewed distribution of the primary endpoints, a nonparametric Mann–Whitney U-test for two unpaired samples were used for comparison. Two-sided p-values with a significance level < 0.05 were used to indicate statistical significance.
In secondary analyses, multiple linear regression was used to quantify the contribution of the availability of the new MEP as a predictor variable (coded as study site SC1 vs study site SC2) of total use of antibiotics and length of hospital stay, respectively, as the outcome variables, adjusted for several preselected covariates. We used stepwise regression with a combined forwards and backwards selection algorithm to optimize the models. Additionally, we calculated generalized variance inflation factors (GVIFs) in order to rule out multicollinearity of predictor variables. We defined \(\surd 5\) as the threshold for multicollinearity [50].
As this study’s sample size was determined by the retrospective study design through availability of full datasets (2017 and 2018), the study’s power was calculated retrospectively. Assuming normally distributed data, the achieved sample size (n1 = 106, n2 = 107) would detect small-to medium effect sizes (Cohen’s d = 0.4) with 82% power; medium effect sizes (Cohen’s d = 0.5) would be detected with 95% power.
The data were analyzed with RStudio, version 1.2.1335 (R Core Team 2017. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL: https://www.R-project.org).
Post-hoc analysis: comparison of MEP vs. no-MEP cases
From the total of 213 patients, 63 were subjected to MEP diagnostics, 150 were not evaluated by the MEP. As expected due to the pre-planned selection of more severely affected patients for MEP diagnostics, the two groups showed reduced comparability, illustrating the selection process (see Table 8): More patients from the MEP group were only in fair/stable state (68.3%) vs. 45.3% in the non-MEP group (descriptive p = 0.004); vice versa, fewer patients from the MEP group were in “good” general state at admission (23.8%) vs. 46.7% in the non-MEP group (descriptive p = 0.003). Additionally, patients from the MEP group showed a trend towards a higher proportion of ICU/NICU admissions (27.0% vs. 15.3%, p = 0.07). There were no relevant differences regarding sex, median age, CrP and body temperature at admission.
All types of intravenous antiinfectives were given for a total of 404 days in the MEP group (n = 63), and for a total of 742 days in the non-MEP group (n = 150). Median exposures per patient were 4.67 days (IQR 0–12.67 d) in the MEP group, and 3.00 days in the non-MEP group (IQR 0–7.81 d). This difference regarding intravenous antiifectives between the two groups thus can be described as relevant (p = 0.04). In the MEP group, the median length of hospital stay per patient was 140.9 h (IQR 87–228 h); in non-MEP group, the median length was 97.2 h (IQR 60.3–163.4 h). As well, the difference between the two groups is relevant (p = 0.02).
Detailed overview of the impact of MEP results on the length of hospital stay
See Appendix Table 9.
Rights and permissions
About this article
Cite this article
Disse, S.C., Zapf, A., Schneble, F. et al. The clinical impact of multiplex PCR panel diagnostics in paediatric meningitis/ encephalitis: a bicenter cohort study. Infection 50, 1329–1348 (2022). https://doi.org/10.1007/s15010-022-01836-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-022-01836-5