Abstract
Purpose
Treatment guidelines often do not advocate testing for integrase inhibitor resistance associated mutations (IRAM) before initiation of first line ART given the extremely low prevalence of mutations found in older surveillance studies. We aimed to describe the prevalence of IRAM in Austrian patients recently diagnosed with HIV in the 5 years following introduction of integrase inhibitors and to analyse trends and factors associated with their detection.
Methods
Samples of antiretroviral treatment (ART) naïve patients recently diagnosed with HIV in Austria between 2008 and 2013 were analysed for the existence of IRAM and drug penalty scores were calculated to estimate response to drugs. Demographic and virological data were extracted from a database. Descriptive and comparative statistics were used.
Results
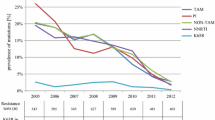
A total of 303 samples were analysed. 78 % were male and mean age was 38 years. Overall prevalence of IRAM was 2.3 %. Six percent had at least potentially low-level resistance to raltegravir or elvitegravir, versus 1 % for dolutegravir. One primary mutation was observed (F121Y) in a patient sample from 2012 leading to 5–10-fold reduced susceptibility to raltegravir and elvitegravir. Two patients carried the accessory mutations E138K and G140A, respectively, where both lie on the Q148 pathway. No temporal trend of IRAM prevalence was observed (p = 0.16).
Discussion
Primary IRAM are still rarely found despite the increasing use of INSTI in Austria, but there is a potential for reduced susceptibility to these drugs in selected patients. Routine resistance testing seems prudent to avoid the consequences including accumulation of further mutations and therapeutic failure.
Similar content being viewed by others
References
Lennox JL, DeJesus E, Lazzarin A, Pollard RB, Madruga JV, Berger DS, et al. Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment-naive patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet. 2009;374:796–806. doi:10.1016/S0140-6736(09)60918-1.
Sax PE, DeJesus E, Mills A, Zolopa A, Cohen C, Wohl D, et al. Co-formulated elvitegravir, cobicistat, emtricitabine, and tenofovir versus co-formulated efavirenz, emtricitabine, and tenofovir for initial treatment of HIV-1 infection: a randomised, double-blind, phase 3 trial, analysis of results after 48 weeks. Lancet. 2012;379:2439–48. doi:10.1016/S0140-6736(12)60917-9.
Oliveira M, Mesplede T, Quashie PK, Moisi D, Wainberg MA. Resistance mutations against dolutegravir in HIV integrase impair the emergence of resistance against reverse transcriptase inhibitors. AIDS. 2014;28:813–9. doi:10.1097/QAD.0000000000000199.
Malet I, Delelis O, Valantin MA, Montes B, Soulie C, Wirden M, et al. Mutations associated with failure of raltegravir treatment affect integrase sensitivity to the inhibitor in vitro. Antimicrob Agents Chemother. 2008;52:1351–8. doi:10.1128/AAC.01228-07.
Cooper DA, Steigbigel RT, Gatell JM, Rockstroh JK, Katlama C, Yeni P, et al. Subgroup and resistance analyses of raltegravir for resistant HIV-1 infection. N Engl J Med. 2008;359:355–65. doi:10.1056/NEJMoa0708978.
Goethals O, Clayton R, Van Ginderen M, Vereycken I, Wagemans E, Geluykens P, et al. Resistance mutations in human immunodeficiency virus type 1 integrase selected with elvitegravir confer reduced susceptibility to a wide range of integrase inhibitors. J Virol. 2008;82:10366–74. doi:10.1128/JVI.00470-08.
Rockstroh JK, DeJesus E, Lennox JL, Yazdanpanah Y, Saag MS, Wan H, et al. Durable efficacy and safety of raltegravir versus efavirenz when combined with tenofovir/emtricitabine in treatment-naive HIV-1-infected patients: final 5-year results from STARTMRK. J Acquir Immune Defic Syndr. 2013;63:77–85. doi:10.1097/QAI.0b013e31828ace69.
Eron JJ, Cooper DA, Steigbigel RT, Clotet B, Gatell JM, Kumar PN, et al. Efficacy and safety of raltegravir for treatment of HIV for 5 years in the BENCHMRK studies: final results of two randomised, placebo-controlled trials. Lancet Infect Dis. 2013;13:587–96. doi:10.1016/S1473-3099(13)70093-8.
Hofstra LM, Sauvageot N, Albert J, Alexiev I, Garcia F, Struck D, et al. Transmission of HIV drug resistance and the predicted effect on current first-line regimens in Europe. Clin Infect Dis. 2015;. doi:10.1093/cid/civ963.
Stekler JD, McKernan J, Milne R, Tapia KA, Mykhalchenko K, Holte S, et al. Lack of resistance to integrase inhibitors among antiretroviral-naive subjects with primary HIV-1 infection, 2007–2013. Antivir Ther. 2015;20:77–80. doi:10.3851/IMP2780.
Saladini F, Meini G, Bianco C, Monno L, Punzi G, Pecorari M, et al. Prevalence of HIV-1 integrase mutations related to resistance to dolutegravir in raltegravir naive and pretreated patients. Clin Microbiol Infect. 2012;18:E428–30. doi:10.1111/j.1469-0691.2012.03917.x.
Gutierrez C, Hernandez-Novoa B, Perez-Elias MJ, Moreno AM, Holguin A, Dronda F, et al. Prevalence of primary resistance mutations to integrase inhibitors in treatment-naive and -experienced patients infected with B and non-B HIV-1 variants. HIV Clin Trials. 2013;14:10–6. doi:10.1410/hct1401-10.
Sichtig N, Sierra S, Kaiser R, Daumer M, Reuter S, Schulter E, et al. Evolution of raltegravir resistance during therapy. J Antimicrob Chemother. 2009;64:25–32. doi:10.1093/jac/dkp153.
Liu TF, Shafer RW. Web resources for HIV type 1 genotypic-resistance test interpretation. Clin Infect Dis. 2006;42:1608–18. doi:10.1086/503914.
Wensing AM, Calvez V, Gunthard HF, Johnson VA, Paredes R, Pillay D, et al. 2015 Update of the drug resistance mutations in HIV-1. Top Antivir Med. 2015;23:132–41.
Kobayashi M, Nakahara K, Seki T, Miki S, Kawauchi S, Suyama A, et al. Selection of diverse and clinically relevant integrase inhibitor-resistant human immunodeficiency virus type 1 mutants. Antiviral Res. 2008;80:213–22. doi:10.1016/j.antiviral.2008.06.012.
(AHIVCOS) AHCS. HIV/AIDS in Austria: 26th report of the Austrian HIV cohort study. Innsbruck: STUDIA Universitätsverlag; 2014.
DAIG. Deutsch-Österreichische Leitlinien zur antiretroviralen Therapie der HIV-Infektion. 2012. http://www.daignet.de/site-content/hiv-therapie/leitlinien-1/Anlage%201%20LL%20055-001%20Version%206%2011-12-2015%20endgultige%20Version%20rev-2.pdf. Accessed 12 Aug 2016.
Souza Cavalcanti J, Minhoto Lanca A, de Paula Ferreira JL, da Eira M, de Souza Dantas DS, de Macedo Brigido LF. In-vivo selection of the mutation F121Y in a patient failing raltegravir containing salvage regimen. Antiviral Res. 2012;95:9–11. doi:10.1016/j.antiviral.2012.04.007.
Munir S, Thierry E, Malet I, Subra F, Calvez V, Marcelin AG, et al. G118R and F121Y mutations identified in patients failing raltegravir treatment confer dolutegravir resistance. J Antimicrob Chemother. 2015;70:739–49. doi:10.1093/jac/dku474.
Metzner KJ, Scherrer AU, Preiswerk B, Joos B, von Wyl V, Leemann C, et al. Origin of minority drug-resistant HIV-1 variants in primary HIV-1 infection. J Infect Dis. 2013;208:1102–12. doi:10.1093/infdis/jit310.
Coffin JM. HIV population dynamics in vivo: implications for genetic variation, pathogenesis, and therapy. Science. 1995;267:483–9.
Casadella M, van Ham PM, Noguera-Julian M, van Kessel A, Pou C, Hofstra LM, et al. Primary resistance to integrase strand-transfer inhibitors in Europe. J Antimicrob Chemother. 2015;70:2885–8. doi:10.1093/jac/dkv202.
Mbisa JL, Fearnhill E, Dunn DT, Pillay D, Asboe D, Cane PA, et al. Evidence of self-sustaining drug resistant HIV-1 lineages among untreated patients in the United Kingdom. Clin Infect Dis. 2015;61:829–36. doi:10.1093/cid/civ393.
Acknowledgments
The performance of this study was financially supported by GILEAD.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Zoufaly, A., Kraft, C., Schmidbauer, C. et al. Prevalence of integrase inhibitor resistance mutations in Austrian patients recently diagnosed with HIV from 2008 to 2013. Infection 45, 165–170 (2017). https://doi.org/10.1007/s15010-016-0936-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s15010-016-0936-5