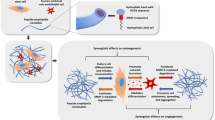
Abstract
Background:
Μonocyte-derived multipotential cells (MOMCs) include progenitors capable of differentiation into multiple cell lineages and thus represent an ideal autologous transplantable cell source for regenerative medicine. In this study, we cultured MOMCs, generated from mononuclear cells of peripheral blood, on the surface of nanocomposite thin films.
Methods:
For this purpose, nanocomposite Poly(e-caprolactone) (PCL)-based thin films containing either 2.5 wt% silica nanotubes (SiO2ntbs) or strontium hydroxyapatite nanorods (SrHAnrds), were prepared using the spin-coating method. The induced differentiation capacity of MOMCs, towards bone and endothelium, was estimated using flow cytometry, real-time polymerase chain reaction, scanning electron microscopy and fluorescence microscopy after cells’ genetic modification using the Sleeping Beauty Transposon System aiming their observation onto the scaffolds. Moreover, Wharton’s Jelly Mesenchymal Stromal Cells were cultivated as a control cell line, while Human Umbilical Vein Endothelial Cells were used to strengthen and accelerate the differentiation procedure in semi-permeable culture systems. Finally, the cytotoxicity of the studied materials was checked with MTT assay.
Results:
The highest differentiation capacity of MOMCs was observed on PCL/SiO2ntbs 2.5 wt% nanocomposite film, as they progressively lost their native markers and gained endothelial lineage, in both protein and transcriptional level. In addition, the presence of SrHAnrds in the PCL matrix triggered processes related to osteoblast bone formation.
Conclusion:
To conclude, the differentiation of MOMCs was selectively guided by incorporating SiO2ntbs or SrHAnrds into a polymeric matrix, for the first time.
Graphical Abstract









Similar content being viewed by others
References
Dawson E, Mapili G, Erickson K, Taqvi S, Roy K. Biomaterials for stem cell differentiation. Adv Drug Deliv Rev. 2008;60:215–28.
Keane TJ, Badylak SF. Biomaterials for tissue engineering applications. Semin Pediatr Surg. 2014;23:112–8.
O’Brien FJ. Biomaterials & scaffolds for tissue engineering. Mater Today (Kidlington). 2011;14:88–95.
Vacanti J. Tissue engineering and regenerative medicine: from first principles to state of the art. J Pediatr Surg. 2010;45:291–4.
Howard D, Buttery LD, Shakesheff KM, Roberts SJ. Tissue engineering: strategies, stem cells and scaffolds. J Anat. 2008;213:66–72.
Chen Y, Shao JZ, Xiang LX, Dong XJ, Zhang GR. Mesenchymal stem cells: a promising candidate in regenerative medicine. Int J Biochem Cell Biol. 2008;40:815–20.
Bianco P. “Mesenchymal” stem cells. Annu Rev Cell Dev Biol. 2014;30:677–704.
Shamsi M, Karimi M, Ghollasi M, Nezafati N, Shahrousvand M, Kamali M, et al. In vitro proliferation and differentiation of human bone marrow mesenchymal stem cells into osteoblasts on nanocomposite scaffolds based on bioactive glass (64SiO2–31CaO–5P2O5)-poly-l-lactic acid nanofibers fabricated by electrospinning method. Mater Sci Eng C Mater Biol Appl. 2017;78:114–23.
Kim DW, Staples M, Shinozuka K, Pantcheva P, Kang SD, Borlongan CV. Wharton’s jelly-derived mesenchymal stem cells: phenotypic characterization and optimizing their therapeutic potential for clinical applications. Int J Mol Sci. 2013;14:11692–712.
Abdallah BM, Kassem M. Human mesenchymal stem cells: from basic biology to clinical applications. Gene Ther. 2008;15:109–16.
Seta N, Kuwana M. Human circulating monocytes as multipotential progenitors. Keio J Med. 2007;56:41–7.
Seta N, Kuwana M. Derivation of multipotent progenitors from human circulating CD14+ monocytes. Exp Hematol. 2010;38:557–63.
Kuwana M, Okazaki Y, Kodama H, Izumi K, Yasuoka H, Ogawa Y, et al. Human circulating CD14+ monocytes as a source of progenitors that exhibit mesenchymal cell differentiation lineages. J Leukoc Biol. 2003;74:833–45.
Kuwana M, Okazaki Y, Kodama H, Satoh T, Kawakami Y, Ikeda Y. Endothelial differentiation potential of human monocyte-derived multipotential cells. Stem Cells. 2006;24:2733–43.
Seta N, Okazaki Y, Miyazaki H, Kato T, Kuwana M. Platelet-derived stromal cell-derived factor-1 is required for the transformation of circulating monocytes into multipotential cells. PLoS One. 2013;8:e74246.
Seta N, Okazaki Y, Izumi K, Miyazaki H, Kato T, Kuwana M. Fibronectin binding is required for acquisition of mesenchymal/endothelial differentiation potential in human circulating monocytes. Clin Dev Immunol. 2012;2012:820827.
Chrissopoulou K, Anastasiadis SH. Effects of nanoscopic-confinement on polymer dynamics. Soft Matter. 2015;11:3746–66.
Peters R. Nanoscopic medicine: the next frontier. Small. 2006;2:452–6.
Grigoriadou I, Nianias N, Hoppe A, Terzopoulou Z, Bikiaris D, Will J, et al. Evaluation of silica-nanotubes and strontium hydroxyapatite nanorods as appropriate nanoadditives for poly(butylene succinate) biodegradable polyester for biomedical applications. Compos B Eng. 2014;60:49–59.
Abedalwafa M, Wang F, Wang L, Li C. Biodegradable poly-epsilon-caprolactone (PCL) for tissue engineering applications: a review. Rev Adv Mater Sci. 2013;34:123–40.
Nerantzaki M, Filippousi M, Van Tendeloo G, Terzopoulou Z, Bikiaris D, Goudouri OM, et al. Novel poly(butylene succinate) nanocomposites containing strontium hydroxyapatite nanorods with enhanced osteoconductivity for tissue engineering applications. Express Polym Lett. 2015;9:773–89.
Nerantzaki M, Kehagias N, Francone A, Fernández A, Torres CMS, Papi R, et al. Design of a multifunctional nanoengineered PLLA surface by maximizing the synergies between biochemical and surface design bactericidal effects. ACS Omega. 2018;3:1509–21.
Elzein T, Nasser-Eddine M, Delaite C, Bistac S, Dumas P. FTIR study of polycaprolactone chain organization at interfaces. J Colloid Interface Sci. 2004;273:381–7.
Chen H, He J. One-step synthesis of functional chiral porous silica nanorods using an achiral surfactant. Dalton Trans. 2009:6651–5.
Xue C, Chen Y, Huang Y, Zhu P. Hydrothermal synthesis and biocompatibility study of highly crystalline carbonated hydroxyapatite nanorods. Nanoscale Res Lett. 2015;10:1018.
Koliakos I, Tsagias N, Karagiannis V. Mesenchymal cells isolation from Wharton’s jelly, in perspective to clinical applications. J Biol Res (Thessalon). 2011;16:194–201.
Kaihara S, Borenstein J, Koka R, Lalan S, Ochoa ER, Ravens M, et al. Silicon micromachining to tissue engineer branched vascular channels for liver fabrication. Tissue Eng. 2000;6:105–17.
Skaat H, Ziv-Polat O, Shahar A, Last D, Mardor Y, Margel S. Magnetic scaffolds enriched with bioactive nanoparticles for tissue engineering. Adv Healthc Mater. 2012;1:168–71.
Tuan RS, Boland G, Tuli R. Adult mesenchymal stem cells and cell-based tissue engineering. Arthritis Res Ther. 2003;5:32–45.
Meng X, Leslie P, Zhang Y, Dong J. Stem cells in a three-dimensional scaffold environment. Springerplus. 2014;3:80.
Patrício T, Domingos M, Gloria A, Bártolo P. Characterisation of PCL and PCL/PLA scaffolds for tissue engineering. Procedia CIRP. 2013;5:110–4.
Wei M, Li S, Le W. Nanomaterials modulate stem cell differentiation: biological interaction and underlying mechanisms. J Nanobiotechnology. 2017;15:75.
Nguyen BB, Moriarty RA, Kamalitdinov T, Etheridge JM, Fisher JP. Collagen hydrogel scaffold promotes mesenchymal stem cell and endothelial cell coculture for bone tissue engineering. J Biomed Mater Res A. 2017;105:1123–31.
Ikada Y. Challenges in tissue engineering. J R Soc Interface. 2006;3:589–601.
Acknowledgements
The authors thank Biohellenika SA Biotechnology Company for providing the facilities and consumables that enabled the completion of this study. EG and GK conceived the study; MN and DB fabricated and characterized the materials; IK and EG designed and performed the experiments (equal contribution to this work); IK, EG, EP, MK and DB analysed the data; IK, EG and MN wrote the manuscript. All authors reviewed the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
This study was approved by the Ethics Committee of Aristotle University of Thessaloniki, School of Medicine (390-9/1.7.2017) and University Hospital AXEPA of Thessaloniki (3868/24.1.2018). This research involves Human Participants after their informed consent. Peripheral blood samples were collected from healthy volunteer donors while mesenchymal stromal cells and HUVEC were isolated from Wharton’s jelly after parents approval during stem cell banking in Biohellenika SA Biotechnology Company.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Koliakou, I., Gounari, E., Nerantzaki, M. et al. Differentiation Capacity of Monocyte-Derived Multipotential Cells on Nanocomposite Poly(e-caprolactone)-Based Thin Films. Tissue Eng Regen Med 16, 161–175 (2019). https://doi.org/10.1007/s13770-019-00185-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-019-00185-z