Abstract
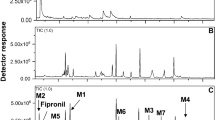
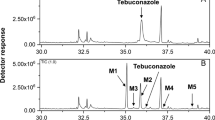
Fenazaquin (4-(2-(4-t-butylphenyl)ethoxy)quinazoline) is a quinazoline insecticide, which contains a rare pesticidal toxophore, quinazoline. Its metabolic fate in animals and plants was previously reported. However, the microbial metabolism of the compound has never been studied. Microbial transformation is an important research area for the investigation of environmental safety issues of pesticides. Aspergillus niger was selected as a model soil fungus since it is ubiquitous in agricultural soils, with extensive genetic studies undertaken. Fenazaquin was rapidly metabolized by A. niger (half-life, t1/2 = 0.6 day). 4-Hydroxyquinazoline and 4-t-butylphenethyl alcohol were identified as major metabolites from the cultures. Fenazaquin was also rapidly transformed into the same metabolites (t1/2 = 0.1–0.5 day) under chemical oxidation (m-chloroperoxybenzoic acid). Among the several metabolic inhibitors, flavin-dependent mono-oxygenase inhibitor, methimazole yielded no inhibitory activity (t1/2 = 1.6 day). Several cytochrome P450 inhibitors including piperonyl butoxide, ketoconazole, and myclobutanil were also tested. Piperonyl butoxide strongly reduced fenazaquin metabolism (t1/2 = 58.7 days). However, ketoconazole and myclobutanil showed no activity even at fungi-toxic concentrations (t1/2 = 1.2–4.3 days) with major metabolites similar to those of control experiments. The results suggest that oxidative metabolism of fenazaquin was catalyzed by specific cytochrome P450s, which are insensitive to azole fungicides. In addition, piperonyl butoxide was found to be one of the most promising synergists of pesticides, through cytochrome P450 inhibition.





Similar content being viewed by others
References
Solomon MG, Fitzgerald JD, Ridout MS (1993) Fenazaquin, a selective acaricide for use in IPM in apple in the UK. Crop Prot 12:255–258
Wood E, Latli B, Casida JE (2003) Fenazaquin acaricide specific binding sites in NADH: ubiquinone oxidoreductase and apparently the ATP synthase stalk. Pestic Biochem Physiol 54:135–145
Hollingworth R, Ahmmadsahib K, Gedelhak G, McLaughlin J (1994) New inhibitors of complex I of the mitochondrial electron transport chain with activity as pesticides. Biochem Soc Trans (Lond.) 22:230–233
Coscolla C, Yusa V, Beser MI, Pastor A (2009) Multi-residue analysis of 30 currently used pesticides in fine airborne particulate matter (PM 2.5) by microwave-assisted extraction and liquid chromatography-tandem mass spectrometry. J Chromatogr A 1216:8817–8827
Tewary DK, Kumar V, Shanker A (2004) Leaching of pesticides in herbal decoction. Chem Health Saf 11:25–29
Roberts TR, Hutson DH, Lee PW, Nicholls PH, Plimmer JR (1999) Metabolic pathways of agrochemicals, part 2: insecticides and fungicides. R Society of Chemistry, London, pp 747–751
Bhattacharyya J, Pramanik SK, Banerjee H, Bhattacharyya A (2010) Effect of pH on the fate of fenazaquin in soil and water under laboratory-simulated condition. Toxicol Environ Chem 92:1083–1092
Kah M, Beulke S, Brown CD (2007) Factors influencing degradation of pesticides in soil. J Agric Food Chem 55:4487–4492
Arbeli Z, Fuentes CL (2007) Accelerated biodegradation of pesticides: an overview of the phenomenon, its basis and possible solutions; and a discussion on the tropical dimension. Crop Prot 26:1733–1746
Maqbool Z, Hussain S, Imran M, Mahmood F, Shahzad T, Ahmed Z, Azeem F, Muzammil S (2016) Perspectives of using fungi as bioresource for bioremediation of pesticides in the environment: a critical review. Environ Sci Pollut Res Int 23:16904–16925
Kamei I, Takagi K, Kondo R (2011) Degradation of endosulfan and endosulfan sulfate by white-rot fungus Trametes hirsuta. J Wood Sci 57:317–322
Purnomo AS, Putra SR, Shimizu K, Kondo R (2014) Biodegradation of heptachlor and heptachlor epoxide-contaminated soils by white-rot fungal inocula. Environ Sci Pollut Res 21:11305–11312
Singh BK, Kuhad RC (1999) Biodegradation of lindane (γ-hexachlorocyclohexane) by the white rot fungus Trametes hirsutus. Lett Appl Microbiol 28:238–241
Badawi N, Rønhede S, Olsson S, Kragelund BB, Johnsen AH, Jacobsen OS, Aamand J (2009) Metabolites of the phenylurea herbicides chlorotoluron, diuron, isoproturon and linuron produced by the soil fungus Mortierella sp. Environ Pollut 157:2806–2812
Fang H, Xiang YQ, Hao YJ, Chu XQ, Pan XD, Yu JQ, Yu YL (2008) Fungal degradation of chlorpyrifos by Verticillium sp. DSP in pure cultures and its use in bioremediation of contaminated soil and pakchoi. Int Biodeterior Biodegr 61:294–303
Hangler M, Jensen B, Rønhede S, Sørensen SR (2007) Inducible hydroxylation and demethylation of the herbicide isoproturon by Cunninghamella elegans. FEMS Microbiol Lett 268:254–260
Pinto AP, Serrano C, Pires T, Mestrinho E, Dias L, Teixeira DM, Caldeira AT (2012) Degradation of terbuthylazine difenoconazole and pendimethalin pesticides by selected fungi cultures. Sci Total Environ 435:402–410
Ueyama I, Araki Y, Kurogochi S, Yamaguchi I (1993) Metabolism of the phenylurea fungicide, pencycuron, in sensitive and tolerant strains of Rhizoctonia solani. J Pestic Sci 18:109–117
Kim YH, Ahn JY, Moon SH, Lee J (2005) Biodegradation and detoxification of organophosphate insecticide, malathion by Fusarium oxysporum f. sp. pisi cutinase. Chemosphere 60:1349–1355
Salama AKM (1998) Metabolism of carbofuran by Aspergillus niger and Fusarium graminearum. J Environ Sci Health B 33:253–266
Liang WQ, Wang ZY, Li H, Wu PC, Hu JM, Luo N, Cao LX, Liu YH (2005) Purification and characterization of a novel pyrethroid hydrolase from Aspergillus niger ZD11. J Agric Food Chem 53:7415–7420
Lim DS, Lim DH, Lee JH, Oh ET, Keum YS (2017) Structure-oxidative metabolism relationships of substituted flavones by Aspergillus niger. J Agric Food Chem 65:3056–3064
Galagan JE, Calvo SE, Cuomo C, Ma LJ, Wortman JR, Batzoglou S, Lee SI, Baştürkmen M, Spevak CC, Clutterbuck J, Kapitonov V, Jurka J, Scazzocchio C, Farman M, Butler J, Purcell S, Harris S, Braus GH, Draht O, Busch S, D’Enfert C, Bouchier C, Goldman GH, Bell-Pedersen D, Griffiths-Jones S, Doonan JH, Yu J, Vienken K, Pain A, Freitag M, Selker EU, Archer DB, Peñalva MA, Oakley BR, Momany M, Tanaka T, Kumagai T, Asai K, Machida M, Nierman WC, Denning DW, Caddick M, Hynes M, Paoletti M, Fischer R, Miller B, Dyer P, Sachs MS, Osmani SA, Birren BW (2005) Sequencing of Aspergillus nidulans and comparative analysis with A. fumigatus and A. oryzae. Nature 438:1105–1115
Sun J, Lu X, Rinas U, Zeng AP (2007) Metabolic peculiarities of Aspergillus niger disclosed by comparative metabolic genomics. Genome Biol 8:R182
Ffrench-Constant RH (2013) The molecular genetics of insecticide resistance. Genetics 194:807–815
Szymanska E, Frydenvang K, Contreras-Sanz A, Pickering DS, Frola E, Serafimoska Z, Nielsen B, Kastrup JS, Johansen TN (2011) A new phenylalanine derivative acts as an antagonist at the AMPA receptor GluA2 and introduces partial domain closure: synthesis, resolution, pharmacology, and crystal structure. J Med Chem 54:7289–7298
Casida JE, Ruzo LO (1986) Reactive intermediates in pesticide metabolism: peracid oxidations as possible biomimetic models. Xenobiotica 16:1003–1015
Keserü GM (1998) Cytochrome P450 catalysed insecticide metabolism: chemical and theoretical models. Sci Progr 81:245–272
Segall Y (2011) Biomimetic chemistry as a useful tool for studying reactive metabolites of pesticides. J Agric Food Chem 59:2845–2856
Hussain H, Al-Harrasi A, Green IR, Ahmed I, Abbasa G, Rehmana NU (2014) meta-Chloroperbenzoic acid (mCPBA): a versatile reagent in organic synthesis. RSC Adv 4:12882–12917
Kamijo S, Matsumura S, Inoue M (2010) CCl3CN: a crucial prometer of mCPBA-mediated direct ether oxidation. Org Lett 12:4195–4197
Chen J, Wong MH, Wong YS, Tam NF (2008) Multi-factors on biodegradation kinetics of polycyclic aromatic hydrocarbons (PAHs) by Sphingomonas sp. a bacterial strain isolated from mangrove sediment. Mar Pollut Bull 57:695–702
Zeng F, Cui K, Li X, Fu J, Sheng G (2004) Biodegradation kinetics of phthalate esters by Pseudomonas fluoresences FS1. Process Biochem 39:1125–1129
Bhattacharyya J, Banerjee H, Bhattacharyya A (2003) Photodecomposition of an acaricide, fenazaquin, in aqueous alcoholic solution. J Agric Food Chem 51:4013–4016
Bhattacharyya J, Banerjee H, Das SP, Bhattacharyya A (2005) Metabolism of fenazaquin, an acaricide in tea plant. Bull Environ Contam Toxicol 75:569–573
Cedergreen N (2014) Quantifying synergy: a systematic review of mixture toxicity studies within environmental toxicology. PLoS ONE 9:e96580. https://doi.org/10.1371/journal.pone.0096580
Gressel J (1993) Synergizing pesticides to reduce use rates. In: Duke SO, Menn JJ, Plimmer JR (eds) Pest control with enhanced environmental safety. ACS symposium series, vol 524, American Chemical Society, Washington, pp 48-61
Venkatakrishnan K, von Moltke LL, Greenblatt DJ (2000) Effects of the antifungal agents on oxidative drug metabolism. Clin Pharmacokinet 38:111–180
Farnham AW (1999) The mode of action of piperonyl butoxide with reference to studying pesticide resistance. In: Jones DG (ed) Piperonyl butoxide the insecticide synergist. Academic Press, San Diego, pp 199–213
Phillips IR, Shephard EA (2017) Drug metabolism by flavin-containing monooxygenases of human and mouse. Expert Opin Drug Metab Toxicol 13:167–181
Hainzl D, Cole LM, Casida JE (1998) Mechanisms for selective toxicity of fipronil insecticide and its sulfone metabolite and desulfinyl photoproduct. Chem Res Toxicol 11:1529–1535
Acknowledgments
This research was supported by the Ministry of Food and Drug Safety.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Joo, SH., Keum, YS. Oxidative metabolism of quinazoline insecticide fenazaquin by Aspergillus niger. Appl Biol Chem 61, 681–687 (2018). https://doi.org/10.1007/s13765-018-0404-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13765-018-0404-2