Abstract
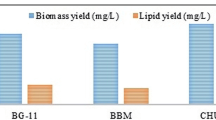
Microalgae have emerged as a potential alternative for the production of many useful compounds like protein, carbohydrate and lipid. Lipid-rich microalgae are important and rich source for alternative energy production. In order to commercially utilize microalgae for energy production, the lipid productivity should be enhanced. Keeping in view the above-mentioned potentials of microalgae, in the present study, we have attempted to display the role of chemical stimulants and light in the growth and lipid production of the microalgae Chlorella singularis (UUIND5). During the present investigations, effect of varying photoperiods and different types of lights and chemical stimulants, viz. CaCl2 and kinetin on growth rate and lipid production, was studied. The maximum growth rate recorded was 166 ± 0.3 mg/L/d, when 0.80 g/l CaCl2 and 0.5 mg/l kinetin were added to Bold’s basal medium. C. singularis was then cultivated in this medium for 14 days under sunlight +LED (10-h sunlight + 14-h LED light) at photoperiod 24-h light/0-h dark. The maximum lipid yield 30.2% of dry wt. was obtained under sunlight +LED. Further, the gas chromatography analysis also showed the presence of fatty acid methyl esters (FAME). FAMEs profile was analyzed according to ASTM D6751 specification. Thus, it was concluded that sunlight +LED at 24-h light/0-h dark (100 μmol photons m−2 s−1) photoperiod with CaCl2 and kinetin is an effective strategy to boost lipid productivity in C. singularis (UUIND5).





Similar content being viewed by others
References
Spolaore P, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101:87–96
Xin L, Hong-ying H, Ke G, Ying-xue S (2010) Effects of different nitrogen and phosphorus concentrations on the growth, nutrient uptake, and lipid accumulation of a freshwater microalga Scenedesmus sp. Bioresour Technol 101:5494–5500
Li Y, Chen YF, Chen P, Min M, Zhou W, Martinez B, Zhu J, Ruan R (2011) Characterization of a microalga Chlorella sp. well adapted to highly concentrated municipal wastewater for nutrient removal and biodiesel production. Bioresour Technol 102:5138–5144
Loera-Quezada MM, Angeles G, Olguín EJ (2011) Effect of irradiance on the cell density, size and lipid accumulation of Neochloris oleoabundans. Rev Latinoam Biotechnol Amb Algal 2:81–92
Lopes EJ, Scoparo CHG, Lacerda LMCF, France TT (2009) Effect of light cycles (night/day) on CO2 fixation and biomass production by microalgae in photobioreactors. Chem Eng Process Process Intensif 1:306–310
Harun I, Yahya L, Chik MN, Kadir NNA, Asyraf M, Pang MZ (2014) Effects of natural light dilution on microalgae growth. Int J Chem Eng Appl 5(2):112–116
Teo CL, Atta M, Bukhari A, Taisir M, Yusuf AM, Idris A (2014) Enhancing growth and lipid production of marine microalgae for biodiesel production via the use of different LED wavelengths. Bioresour Technol 162:38–44
Cuaresma M, Janssen M, Vílchez C, Wijffels RH (2009) Productivity of Chlorella sorokiniana in a short light-path (SLP) panel photobioreactor under high irradiance. Biotechnol Bioeng 104:352–359
Mahdavian K, Ghorbanli M, Kalantari KM (2008) The effects of ultraviolet radiation on the contents of chlorophyll, flavonoid, anthocyanin and proline in Capsicum annuum L. Turk J Bot 32:25–33
Jordan BR (1996) The effects of ultraviolet-B radiation on plants: a molecular perspective. Adv Bot Res 22:97–162
Guarnieri MT, Nag A, Yang S, Pienkos PT (2013) Proteomic analysis of Chlorella vulgaris: potential targets for enhanced lipid accumulation. J Proteome 93:245–253
Tale M, Ghosh S, Kapadnis B, Kale S (2014) Isolation and characterization of microalgae for biodiesel production from Nisargruna biogas plant effluent. Bioresour Technol. https://doi.org/10.1016/J.Biortech.2014.06.017
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Bhola VR, Desikan SK, Santosh K, Subburamu E, Sanniyasi Bux F (2011) Effects of parameters affecting biomass yield and thermal behaviour of Chlorella vulgaris. J Biosci Bioeng 111:377–382
Kumar V, Nanda M, Verma M (2017) Application of agar liquid-gel transition in cultivation and harvesting of microalgae for biodiesel production. Bioresour Technol 243:163–168
Bligh EG, Dyer WJ (1959) A rapid method for total lipid extraction and purification. Can J Biochem Phys 37:911–917
Patel A, Sindhu DK, Arora N, Singh RP, Pruthi V, Pruthi PA (2015) Biodiesel production from non-edible lignocellulosic biomass of Cassia fistula L. fruit pulp using oleaginous yeast Rhodosporidium kratochvilovae HIMPA1. Bioresour Technol 197:91–98
AOCS (American Oil Chemical Society) (1998) Official methods and recommended practices of AOCS, 5th edn. American Oil Chemical Society, Champaign
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Slocombe PS, Ross M, Thomas N, McNeill S, Stanley SM (2013) A rapid and general method for measurement of protein in micro-algal biomass. Biores Technol 129:51–57. https://doi.org/10.1016/j.biortech.2012.10.163
Kim TH, Yunhee L, Su-Hyun H, Sun-Jin H (2013) The effects of wavelength and wavelength mixing ratios on microalgae growth and nitrogen, phosphorus removal using Scenedesmus sp. for wastewater treatment. Bioresour Technol 130:75–80
Rendón SM, Roldan GJC, Voroney RP (2013) Effect of carbon dioxide concentration on the growth response of Chlorella vulgaris under four different led illumination. Int J Biotechnol Wellness Ind 2:125–131
Wang CY, Fu CC, Liu YC (2007) Effects of using light-emitting diodes on the cultivation of Spirulina platensis. Biochem Eng J 37:21–25
Darko E, Heydarizadeh P, Schoefs B, Sabzalian MR (2014) Photosynthesis under artificial light: the shift in primary and secondary metabolism. Phil Trans R Soc Lond B. https://doi.org/10.1098/rstb.2013.0243
Katsuda T, Shiraishi H, Ishizu N, Ranjbar R, Katoh S (2008) Effect of light intensity and frequency of flashing light from blue light emitting diodes on astaxanthin production by Haematococcus pluvialis. J Biosci Bioeng 105:216–220
Posten C (2009) Design principles of photo-bioreactors for cultivation of microalgae. Eng Life Sci 9:165–177
Wahidin S, Idris A, Shaleh SRM (2013) The influence of light intensity and photoperiod on the growth and lipid content of microalgae Nannochloropsis sp. Bioresour Technol 129:7–11
Xu C, Li X, Zhang L (2013) The effect of calcium chloride on growth, photosynthesis, and antioxidant responses of Zoysia japonica under drought conditions. PLoS ONE 8(7):e68214. https://doi.org/doi.org/10.1371/journal.pone.0068214
Cousson A (2009) Involvement of phospholipase C-independent calcium-mediated abscisic acid signaling during Arabidopsis response to drought. Biol Plant 53:53–62
Sabir S, Asghar HN, Kashif SUR, Khan MY, Akhtar MJ (2013) Synergistic effect of plant growth promoting Rhizobacteria and kinetin on maize. J Anim Plant Sci 23:1750–1755
Sadak SM, Dawood GM, Bakry BA, El-Karamany MF (2013) Synergistic effect of indole acetic acid and kinetin on performance, some biochemical constituents and yield of Faba bean plant grown under newly reclaimed sandy soil. World J. Agric. Sci 9(4):335–344
McKendry P (2002) Energy production from biomass (part 1): overview of biomass. Bioresour Technol 83:37–46
Hartmann A, Albert A, Ganzera M (2015) Effects of elevated ultraviolet radiation on primary metabolites in selected alpine algae and cyanobacteria. J Photochem Photobiol B Biol 149:149–155
Cohen Z (1999) Porphyridium cruentum. In: Cohen Z (ed) Chemicals from microalgae. CRC Press, Boca Raton, pp 1–24
Kumar V, Nanda M, Joshi HC, Singh A, Sharma S, Verma M (2018) Production of biodiesel and bioethanol using algal biomass harvested from fresh water river. Renew Energy 116:606–612
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, V., Kumar, R., Rawat, D. et al. Synergistic dynamics of light, photoperiod and chemical stimulants influences biomass and lipid productivity in Chlorella singularis (UUIND5) for biodiesel production. Appl Biol Chem 61, 7–13 (2018). https://doi.org/10.1007/s13765-017-0332-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13765-017-0332-6