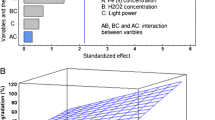
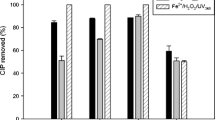
Abstract
This study evaluates the effectiveness of coupling a photo-Fenton process with a biological treatment on the mineralization of enoxacin, an antibacterial agent belonging to the fluoroquinolones group. The influence of some operating parameters, such as UV light intensity, hydrogen peroxide and Fe(II) concentrations, on 50 mg L−1 enoxacin degradation and by-products mineralization was evaluated. The biodegradability of the target molecule was also investigated, reporting a BOD5 on COD ratio of 0.95 after 60 min of irradiation at 15 mmol L−1 of H2O2, 0.5 mmol L−1 of Fe(II) and 30 W m−2 of UV A light intensity. The monitoring of the advanced oxidation state (AOS) of the irradiated solution during the treatment displayed a maximal oxidation state of 0.25 for 90 min of photo-degradation. A comparison between the photo-Fenton process and other oxidative processes [UV alone, UV/H2O2, H2O2, H2O2/Fe(II)] was carried out showing a marked improvement in enoxacin mineralization while combining UV A light with the Fenton reagent (41% of TOC decay improvement compared to the Fenton process). Finally, activated sludge culture for non-treated and pre-treated enoxacin at optimal conditions was conducted during 10 days. The obtained TOC results reported a mineralization improvement with a maximal mineralization yield of 43% for the biodegradation of irradiated samples. The relevance of coupling the photo-Fenton process with a biological process for the enoxacin treatment was therefore proven.









Similar content being viewed by others
References
Ahmed B, Limem E, Abdel-Wahab A, Nasr B (2011) Photo-Fenton treatment of actual agro-industrial wastewaters. Ind Eng Chem Res 50:6673–6680
Alonso Salles N, Fourcade F, Geneste F, Floner D, Amrane A (2010) Relevance of an electrochemical process prior to a biological treatment for the removal of an organophosphorous phosmet. J Hazard Mater 181:617–623
Annabi C, Fourcade F, Soutrel I, Geneste F, Floner D, Bellakhal N, Amrane A (2016) Degradation of enoxacin antibiotic by the electro-Fenton process: optimization, biodegradability improvement and degradation mechanism. J Environ Manag 165:96–105
Assadi AA, Bouzaza A, Wolbert D (2012) Photocatalytic oxidation of trimethylamine and isovaleraldehyde in an annular reactor: influence of the mass transfer and the relative humidity. J Photochem Photobiol A Chem 236:61–69
Babic S, Perisa M, Skoric I (2013) Photolytic degradation of norfloxacin, enrofloxacin and ciprofloxacin in various aqueous media. Chemosphere 91:1635–1642
Baxendale JH, Wilson JA (1957) The photolysis of hydrogen peroxide at high light intensities. Trans Faraday Soc 53:344–356
Bobu M, Yediler A, Siminiceanu I, Zhang F, Schulte-Hostede S (2013) Comparison of different advanced oxidation processes for two fluoroquinolone antibiotics in aqueous solutions. J Environ Sci Health, Part A 48:251–262
Chebli D, Fourcade F, Brosillon S, Nacef S, Amrane A (2010) Supported photocatalysis as a pre-treatment prior to biological degradation for the removal of some dyes from aqueous solutions; Acid Red 183, Biebrich Scarlet, Methyl Red Sodium Salt, Orange II. J Chem Technol Biotechnol 85:555–563
Chen M, Chu W (2012) Degradation of antibiotic norfloxacin in aqueous solution by visible-light mediated C–TiO2 photocatalysis. J Hazard Mater 219–220:183–189
Dal Bosco SM, Barbosa IM, Candello FP, Maniero MG, Rath S, Guimaraès JR (2011) Degradation of ivermectin by Fenton and photo-Fenton and toxicity test using Daphnia similis. J Adv Oxid Technol 14:292–301
Da Silva CR, Maniero MG, Rath S, Guimares JR (2011) Antibacterial activity inhibition after the degradation of flumequine by UV/H2O2. J Adv Oxid Technol 14:106–114
Daughton C, Ternes T (1999) Pharmaceutical and personal care products in the environment: agents of subtle change? Environ Health Perspect 107:907–938
Ekaterina N, Kadnikova N, Kostic M (2002) Oxidation of ABTS by hydrogen peroxide catalyzed by horseradish peroxidase encapsulated into sol–gel glass. Effects of glass matrix on reactvity. J Mol Catal B Enzym 18:39–48
Farré MJ, Franch MI, Ayllón JA, Peral J, Domenech X (2007) Biodegradability of treated aqueous solutions of biorecalcitrant pesticides by means of photocatalytic ozonation. Desalination 211:22–33
Ferrag-Siagh F, Fourcade F, Soutrel I, Aït-Amar H, Djelal H, Amrane A (2013) Tetracycline degradation and mineralization by the coupling of an electro-Fenton pretreatment and a biological process. J Chem Technol Biotechnol 88:1380–1386
Ferrag-Siagh F, Fourcade F, Soutrel I, Aït-Amar H, Djelal H, Amrane A (2014) Electro-Fenton pretreatment for the improvement of tylosin biodegradability. Environ Sci Pollut Res 21:8534–8542
Flox C, Garrido JA, Rodriguez RM, Cabot P, Centellas F, Arias C, Brillas E (2007) Mineralization of herbicide mecoprop by photoelectro-Fenton with UVA and solar light. Catal Today 129:29–36
Fontmorin JM, Huguet S, Fourcade F, Geneste F, Floner D, Amrane A (2012) Electrochemical oxidation of 2,4-dichlorophenoxyacetic acid: analysis of by-products and improvement of the biodegradability. Chem Eng J 195–196:208–217
Fontmorin JM, Fourcade F, Geneste F, Soutrel I, Floner D, Amrane A (2015) Direct electrochemical oxidation of a pesticide, 2,4-dichlorophenoxyacetic acid, at the surface of a graphite felt electrode: biodegradability improvement. C R Chim 18:32–38
Garcia-Segura S, Garrido JA, Rodriguez RM, Cabot PI, Centellas F, Arias C, Brillas E (2012) Mineralization of flumequine in acidic medium by electro-Fenton and photoelectro-Fenton processes. Water Res 46:2067–2076
Giger W, Alder AC, Golet EM, Kohler HPE, McArdell CS, Molnar E, Siegrist H, Suter MJF (2003) Occurrence and fate of antibiotics as trace contaminants in wastewaters, sewage sludges, and surface waters. Chim Int J Chem 57:485–491
Goel RK, Flora JRV, Ferry J (2003) Mechanisms for naphthalene removal during electrolytic aeration. Water Res 37:891–901
Gogate PR, Pandit AB (2004) A review of imperative technologies for wastewater treatment I: oxidation technologies at ambient conditions. Adv Environ Res 8:501–551
Golet EM, Alder AC, Giger W (2002) Environmental exposure and risk assessment of fluoroquinolone antibacterial agents in wastewater and river water of the Gatt Valley Watershed, Switzerland. Environ Sci Technol 36:3645–3651
Guinea E, Garrido JA, Rodriguez RM, Cabot PL, Arias C, Centellas F, Brillas E (2010) Degradation of the fluoroquinolone enrofloxacin by electrochemical advanced oxidation processes based on hydrogen peroxide electrogeneration. Electrochim Acta 55:2101–2115
Halling-Soresen B, Nielson S, Lanzky PF, Ingerslev F, Lutzhoft H, Jorgensen SE (1998) Occurence, fate and effects of pharmaceutical substances in the environment: a review. Chemosphere 36:357–393
Hendricks R, Pool EJ (2012) The effectiveness of sewage treatment processes to remove faecal pathogens and antibiotic residues. J Environ Sci Health, Part A 47:289–297
Kang YW, Hwang KY (2000) Effects of reaction conditions on the oxidation efficiency in the Fenton process. Water Res 34:2786–2790
Kumar A, Paliwal M, Ameta R, Ameta SC (2007) Oxidation of Fast Green FCF by the solar photo-Fenton process. J Iran Chem Soc 5:346–351
Lapertot M, Ebrahimi S, Dazio S, Rubinelli A, Pulgarin C (2007) Photo-Fenton and biological integrated process for degradation of a mixture of pesticides. J Photochem Photobiol A Chem 186:34–40
Lin AC, Yu TH, Lin CF (2008) Pharmaceutical contamination in residential, industrial, and agricultural waste streams: risk to aqueous environments in Taiwan. Chemosphere 74:131–141
Lindberg RH, Olofsson U, Rendahl P, Johansson MI, Tysklind M, Andersson BAV (2006) Behavior of Fluoroquinolones and Trimethoprim during mechanical, chemical and active sludge treatment of sewage water and digestion of sludge. Environ Sci Technol 40:1042–1048
Mansour D, Fourcade F, Bellakhal N, Dachraoui M, Hauchard D, Amrane A (2012) Biodegradability improvement of sulfamethazine solutions by means of an electro-Fenton process. Water Air Soil Pollut 223:2023–2034
Mansour D, Fourcade F, Soutrel I, Hauchard D, Bellakhal N, Amrane A (2015) Relevance of a combined process coupling electro-Fenton and biological treatment for the remediation of sulfamethazine solutions: application to an industrial pharmaceutical effluent. C R Chim 18:39–44
Michael I, Hapeshi C, Michael C, Fatta-Kassinos D (2010) Solar Fenton and solar TiO2 catalytic treatment of ofloxacin in secondary treated effluents: evaluation of operational and kinetic parameters. Water Res 44:5450–5462
Nogueira RFP, Trovo AG, Da Silva MRA, Villa RD, De Oliveira MC (2007) Fundaments and environmental applications of Fenton and photo-Fenton processes. Quim Nova 30:400–408
Parra S, Sarria V, Malato S, Péringer P, Pulgarin C (2000) Photochemical versus coupled photochemical–biological flow system for the treatment of two biorecalcitrant herbicides: metobromuron and isoproturon. Appl Catal B Environ 27:153–168
Parsons S (2004) Advanced oxidation processes for water and wastewater treatment. IWA Publishing, Alliance House, London, p 356
Patel SS, Spencer CM (1996) Enoxacin: a reappraisal of its clinical efficacy in the treatment of genitourinary tract infections. Drugs 51:137–160
Pouran SR, Abdul Aziz AR, Wan Daud WMA (2015) Review on the main advances in photo-Fenton oxidation system for recalcitrant wastewaters. J Ind Eng Chem 21:53–69
Pignatello JJ, Oliveros E, MacKay A (2006) Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit Rev Environ Sci Technol 3:61–84
Pulgarin C, Invernizzi M, Parra S, Sarria V, Polania R, Péringer P (1999) Strategy for the coupling of photochemical and biological flow reactors useful in mineralization of biorecalcitrant industrial pollutants. Catal Today 54:341–352
Rodrigues-Silva C, Maniero MG, Rath S, Guimaràes JR (2013) Degradation of flumequine by the Fenton and the photo-Fenton processes: evaluation of residual antimicrobial activity. Sci Total Environ 445–446:337–346
Romero V, Gonzâlez O, Bayarri B, Pilar M, Gimenez J, Esplugas S (2016) Degradation of metoprolol by photo-Fenton: comparison of different photoreactors performance. Chem Eng J 283:639–648
Rozas O, Contreras D, Mondaca MA, Pérez-Moya M, Mansilla HD (2010) Experimental design of Fenton and photo-Fenton reactions for the treatment of ampicillin solutions. J Hazard Mater 177:1025–1030
Santoke H, Tong AYC, Mezyk SP, Johnston KM, Braund R, Cooper WJ, Peake BM (2015) UV photo-degradation of enoxacin in water: kinetics and degradation pathways. J Environ Eng 141:1–7
Sarria V, Parra S, Adler N, Péringer P, Benitez N, Pulgarin C (2002) Recent developments in the coupling of photoassisted and aerobic biological processes for the treatment of biorecalcitrant compounds. Catal Today 76:301–315
Shemer H, Kunukcu YK, Linden KG (2006) Degradation of the pharmaceutical metronidazole via UV, Fenton and photo-Fenton processes. Chemosphere 63:269–276
Sirés I, Arias C, Cabot PI, Centellas F, Garrido JA, Rodriguez RM, Brillas E (2007) Degradation of clofibric acid in acidic aqueous medium by electro-Fenton and photoelectro-Fenton. Chemosphere 66:1660–1669
Sirtori C, Zapata A, Malato S, Aguera A (2012) Formation of chlorinated by-products during photo-Fenton degradation of pyrimethanil under saline conditions. Influence on toxicity and biodegradability. J Hazard Mater 217–218:217–223
Sirtori C, Zapata A, Malato S, Gernjak W, Fernandez-Alba AR, Aguera A (2009) Solar photocatalytic treatment of quinolones: intermediates and toxicity evaluation. Photochem Photobiol Sci 8:644–651
Tamimi M, Qourzal S, Barka N, Assabane A, Ait-Ichou Y (2008) Methomyl degradation in aqueous solutions by Fenton’s reagent and the photo-Fenton system. Sep Purif Technol 61:103–108
Van Doorslaer X, Dewulf J, Van Langenhove HD, Demeestere K (2014) Fluoroquinolone antibiotics: an emerging class of environmental micropollutants. Sci Total Environ 500–501:250–269
Van Doorslaer X, Haylamicheal ID, Dewulf J, Langenhove HV, Janssen CR, Demeestere K (2015) Heterogeneous photocatalysis of moxifloxacin in water: chemical transformation and ecotoxicity. Chemosphere 119:575–580
Vasconcelos TG, Henriques DM, König A, Martins AF, Kümmerer K (2009) Photo-degradation of the antimicrobial ciprofloxacin at high pH: identification and biodegradability assessment of the primary by-products. Chemosphere 76:487–493
Watkinson AJ, Murby EJ, Costanzo SD (2007) Removal of antibiotics in conventional and advanced wastewater treatment: implications for environmental discharge and wastewater recycling. Water Res 41:4164–4176
Xiao R, He Z, Diaz-Rivera D, Pee GY, Weavers LK (2014) Sonochemical degradation of ciprofloxacin and ibuprofen in the presence of matrix organic compounds. Ultrason Sonochem 21:428–435
Xu XR, Li XY, Li XZ, Li HB (2009) Degradation of melatonin by UV, UV/H2O2, Fe2+/H2O2, UV/Fe2+/H2O2 processes. Sep Purif Technol 68:261–266
Yahiat S, Fourcade F, Brosillon S, Amrane A (2011) Removal of antibiotics integrated process coupling photocatalysis and biological treatment-case of tetracycline and tylosin. Int Biodeterior Biodegrad 65:997–1003
Yahya MS, Oturan N, El Kacemi K, El Karbane M, Aravindakumar CT, Oturan MA (2014) Oxidative degradation study on antimicrobial agent ciprofloxacin by electro-Fenton process: kinetics and oxidation products. Chemosphere 117:447–454
Yoshida M, Lee BD, Hosomi M (2000) Decomposition of aqueous tetrachloroethylene by Fenton oxidation treatment. Water Sci Technol 42:203–208
Zhang Y, Pagilla K (2010) Treatment of malathion pesticide wastewater with nanofiltration and photo-Fenton oxidation. Desalination 263:36–44
Acknowledgements
This work was supported by the Tunisian Ministry of Higher Education and Scientific Research through training fellowships for Miss C. Annabi during her PhD thesis. The authors wish to thank it.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: Q. Aguilar-Virgen.
Electronic supplementary material
Below is the link to the electronic supplementary material.
13762_2018_1701_MOESM3_ESM.tif
ENO chromatograms obtained before (a) and after degradation by the photo-Fenton process during 20 min (b). [ENO]0= 50 mg L-1; [Fe(II)]= 0.5 mmol L-1 ; I= 30 W m-2; pH= 3
13762_2018_1701_MOESM4_ESM.tif
Hydrogen peroxide doses effect on hydrogen peroxide effectiveness (% η) for removing COD by the PF process. [ENO]0= 50 mg L-1; [Fe(II)]= 0.2 mmol L-1 ; I= 30 W m-2; pH= 3; treatment time= 120 min
Rights and permissions
About this article
Cite this article
Annabi, C., Abou Dalle, A., Fourcade, F. et al. Enoxacin degradation by photo-Fenton process combined with a biological treatment: optimization and improvement of by-products biodegradability. Int. J. Environ. Sci. Technol. 16, 655–666 (2019). https://doi.org/10.1007/s13762-018-1701-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-018-1701-3