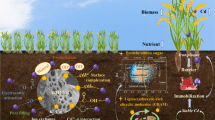
Abstract
Treatment of acid mine drainage (AMD) highly rich in sulfate and multiple metal elements has been investigated in a continuous flow column experiment using organic and inorganic reactive media. Treatment substrates that composed of spent mushroom compost (SMC), limestone, activated sludge and woodchips were incorporated into bacterial sulfate reduction (BSR) treatment for AMD. SMC greatly assisted the removals of sulfate and metals and acted as essential carbon source for sulfate-reducing bacteria (SRB). Alkalinity produced by dissolution of limestone and metabolism of SRB has provided acidity neutralization capacity for AMD where pH was maintained at neutral state, thus aiding the removal of sulfate. Fe, Pb, Cu, Zn and Al were effectively removed (87–100%); however, Mn was not successfully removed despite initial Mn reduction during early phase due to interference with Fe. The first half of the treatment was an essential phase for removal of most metals where contaminants were primarily removed by the BSR in addition to carbonate dissolution function. The importance of BSR in the presence of organic materials was also supported by metal fraction analysis that primary metal accumulation occurs mainly through metal adsorption onto the organic matter, e.g., as sulfides and onto Fe/Mn oxides surfaces.







Similar content being viewed by others
References
Abdullah MH, Kim KW, Aris AZ, Budin K, Praveena SM (2008) Profiling toxic metals distribution in river environment from post-mining area of Mamut, Sabah, Malaysia. Research Report 2008, Universiti Malaysia Sabah
Alshaebi FY, Yaacob WZW, Samsudin AR, Alsabahi E (2009) Risk assessment at abandoned tin mine in Sungai Lembing, Pahang. Electron J Geotech Eng 14:1–9
Ayala-Parra P, Sierra-Alvarez R, Field JA (2016) Treatment of acid rock drainage using a sulfate-reducing bioreactor with zero-valent iron. J Hazard Mater 308:97–105
Bai H, Kang Y, Quan H, Han Y, Sun J, Feng Y (2013) Treatment of acid mine drainage by sulfate reducing bacteria with iron in bench scale runs. Bioresour Technol 128:818–822
Balintova M, Holub M, Singovszka E (2012) Study of iron, copper and zinc removal from acidic solutions by sorption. Chem Eng Trans 28:175–180
Behum PT, Lefticariu L, Bender KS, Segid YT, Burns AS (2011) Remediation of coal-mine drainage by a sulfate-reducing bioreactor: a case study from the Illinois coal basin, USA. Appl Geochem 26:S162–S166
ChaguÉ-Goff C (2005) Assessing the removal efficiency of Zn, Cu, Fe and Pb in a treatment wetland using selective sequential extraction: a case study. Water Air Soil Pollut 160:161–179
Chen H, Wang X, Li J, Wang X (2015) Cotton derived carbonaceous aerogels for the efficient removal of organic pollutants and heavy metal ions. J Mater Chem A 3:6073–6081
Cheong Y, Das B, Roy A, Bhattacharya J (2010) Performance of a SAPS-based chemo-bioreactor using low-DOC spent mushroom compost, and limestone as substrate. Mine Water Environ 29:217–224
Duan S, Liu X, Wang Y, Shao D, Alharbi N, Alsaedi A, Li J (2016) Highly efficient entrapment of U(IV) by porous magnetic Ni0.6Fe2.4O4 micro-particles as the adsorbent. J Taiwan Inst Chem Eng 65:367–377
Dvorak DH, Hedin RS, Edenborn HM, McIntire PE (1992) Treatment of metal-contaminated water using bacterial sulfate reduction: results from pilot-scale reactors. Biotechnol Bioeng 40:609–619
Genty T, Bussiere B, Potvin R, Benzaazoua M, Zagury GJ (2011) Dissolution of calcitic marble and dolomitic rock in high ion concentrated acid mine drainage: application to anoxic limestone drains. Environ Earth Sci 66:2387–2401
Goldani E, Moro C, Maia S (2013) A study employing different clays for Fe and Mn removal in the treatment of acid mine drainage. Water Air Soil Pollut 224:1401–1412
Jena V, Gupta S, Dhundhel RS, Matic N, Bilinski SF, Devic N (2013) Determination of total heavy metal by sequential extraction from soil. Int J Res in Environ Sci Technol 3:35–38
Johnson D, Hallberg K (2005) Acid mine drainage remediation options: a review. Sci Total Environ 3:3–14
Jopony M, Tongkul F (2009) Acid mine drainage at Mamut Copper Mine, Sabah, Malaysia. Borneo Sci 3:83–94
Kijjanapanich P, Annachhatre AP, Esposito G, Lens PNL (2014) Use of organic substrates as electron donors for biological sulfate reduction in gypsiferuos mine soils from Nakhon Si Thammarat (Tahiland). Chemosphere 101:1–7
Kim G, Kim D, Kang J, Baek H (2014) Treatment of synthetic acid mine drainage using rice wine waste as a carbon source. Environ Earth Sci 71:4603–4609
Kusin FM, Zahar MSM, Muhammad SN, Mohamad ND, Zin ZM, Sharif SM (2016a) Hybrid off-river augmentation system as an alternative raw water resource: the hydrogeochemistry of abandoned mining ponds. Environ Earth Sci 75:1–15
Kusin FM, Muhammad SN, Zahar MSM, Zin ZM (2016b) Integrated River Basin Management: incorporating the use of abandoned mining pool and implication on water quality status. Desalin Water Treat 57(60):29126–29136
Lefticariu L, Walters ER, Pugh CW, Bender KS (2015) Sulfate reducing bioreactor dependence on organic substrates for remediation of coal-generated acid mine drainage: field experiments. Appl Geochem 63:70–82
Liu C, Bai R, Ly QS (2008) Selective removal of copper and lead ions by diethylenetriamine-functionalized adsorbent: behaviors and mechanisms. Water Res 42:1511–1522
Liu X, Huang Y, Duan S, Wang Y, Li J, Chen Y, Hayat T, Wang X (2016) Graphene oxides with different oxidation degrees for Co(II) ion pollution management. Chem Eng J 302:763–772
Márquez-Reyes JM, López-Chuken UJ, Valdez-González A, LunaOlvera HA (2013) Removal of chromium and lead by a sulfate reducing consortium using peat moss as carbon source. Bioresour Technol 144:128–134
Mandadi K (2012) Removal of heavy metals using modified limestone media: zinc and cadmium. Western Kentucky University, United States
Mayes W, Davis J, Silva V, Jarvis AP (2011) Treatment of zinc-rich acid mine water in low residence time bioreactors incorporating waste shells and methanol dosing. J Hazard Mater 193:279–287
Muhammad SN, Kusin FM, Zahar MSM, Mohamat-Yusuff F, Halimoon N (2015) Passive treatment of acid mine drainage using mixed substrates: batch experiments. Procedia Environ Sci 30:157–161
Muhammad SN, Kusin FM, Zahar MSM, Mohamat-Yusuff F, Halimoon N (2017) Passive bioremediation technology incorporating lignocellulosic spent mushroom compost and limestone for metal- and sulphate-rich acid mine drainage. Environ Technol 38(16):2003–2012
Neculita C, Zagury G, Bussiere B (2007) Passive treatment of acid mine drainage in bioreactors using sulfate-reducing bacteria: critical review and research needs. J Environ Qual 36:1–16
Sawyer CN, McCarty PL, Parkin GF (2003) Chemistry for Environmental Engineering and Science, 5th edn. McGraw-Hill International, New York
Shi T, Yang D, Feng Y, Song W, Ye J, Zhou Y, Qiu R (2015) Simulative applied study on treatment of acid mine drainage by successive alkalinity producing systems. Chin J Environ Eng 9:2277–2283
Song H, Yim G, Ji S, Neculita CM, Hwang T (2012) Pilot-scale passive bioreactors for the treatment of acid mine drainage: efficiency of mushroom compost vs. mixed substrates for metal removal. J Environ Manag 111:150–158
Tessier A, Campbell PGC, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51:844–851
Vasquez Y, Escobar MC, Neculita CM, Arbeli Z, Roldan F (2016) Biochemical passive reactors for treatment of acid mine drainage: effect of hydraulic residence time on changes in efficiency, composition of reactive mixture, and microbial activity. Chemosphere 153:244–253
Wakeman KD, Erving L, Riekkola-Vanhanen ML, Puhakka JA (2010) Silage supports sulfate reduction in the treatment of metals- and sulfate-containing waste waters. Water Res 44:4932–4939
Wali A, Colinet G, Ksibi M (2014) Speciation of heavy metals by modified BCR sequential extraction in soils contaminated by phosphogypsum in Sfax, Tunisia. Environ Res Eng Manag 4:14–26
Yaacob WZ, Pauzi NSM, Mutalib H (2009) Acid mine drainage and heavy metals contamination at abandoned and active mine sites in Pahang. Bull Geol Soc Malays 55:15–20
Zahar MSM, Kusin FM, Muhammad SN (2015) Adsorption of manganese in aqueous solution by steel slag. Procedia Environ Sci 30:145–150
Zhang S, Zeng M, Li J, Li J, Xu J, Wang X (2014) Porous magnetic carbon sheets from biomass as an adsorbent for the fast removal of organic pollutants from aqueous solution. J Mater Chem A 2:4391–4397
Zhang M, Wang H, Han X (2016) Preparation of metal-resistant immobilized sulfate reducing bacteria beads for acid mine drainage treatment. Chemosphere 154:215–223
Zvinowanda CM, Okonkwo JO, Sekhula MM, Agyei NM, Sadiku R (2009) Application of maize tassel for the removal of Pb, Se, Sr, U and V from borehole water contaminated with mine wastewater in the presence of alkaline metals. J Hazard Mater 164:884–891
Acknowledgements
The authors would like to thank the technical staffs of the Minerals and Geoscience Department, Perak, Imerys Minerals Malaysia Sdn. Bhd and laboratory assistants of Faculty of Environmental Studies, Universiti Putra Malaysia, for their technical assistance during the course of the project.
Funding
This work was primarily supported by Universiti Putra Malaysia under the research Project No. GP-IPS/2014/9438721. The authors also thank the support from research Grant No. FRGS 5524757 (Malaysian Ministry of Higher Education), IPM 9433300 and TWAS-Comstech UNESCO FR:3240270866.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibiility: Josef Trögl.
Rights and permissions
About this article
Cite this article
Muhammad, S.N., Kusin, F.M. & Madzin, Z. Coupled physicochemical and bacterial reduction mechanisms for passive remediation of sulfate- and metal-rich acid mine drainage. Int. J. Environ. Sci. Technol. 15, 2325–2336 (2018). https://doi.org/10.1007/s13762-017-1594-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-017-1594-6