Abstract
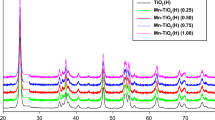
Transition metal-doped TiO2 nanoparticles are synthesized by sol–gel method. The as-prepared samples are characterized by various techniques to correlate structural and optical properties with chemical nature of dopants and their effect on photocatalytic degradation of diethyl phthalate esters. X-ray diffraction (XRD) reveals that all the samples are crystalline and exhibit anatase as a major phase. Chemical nature of dopants could not affect the formation of anatase and its volume fraction. The crystallite size of undoped and doped TiO2 nanoparticles varies between 10 and 12 nm as confirmed by XRD and transmission electron microscope. The lowest optical band gap observed is 2.47 eV in Mn-doped TiO2. Among all the samples, Ni-doped TiO2 sample shows better photocatalytic activity and degradation of diethyl phthalate due to its lower crystallite size and higher surface area than those of Mn- and Co-doped TiO2 samples.










Similar content being viewed by others
References
Altin I, Sokmen M, Biyiklioglu Z (2016) Sol gel synthesis of cobalt doped TiO2 and its dye sensitization for efficient pollutant removal. Mater Sci Semicond Process 45:36–44
Chen X, Liu L, Yu PY, Mao SS (2011) Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 331:746–750
Cheng TS (2012) The toxic effects of diethyl phthalate on the activity of glutamine synthetase in greater duckweed (Spirodela polyrhiza L.). Aquat Toxicol 124–12:171–178
Choi W, Termin A, Hoffmann MR (1994) The role of metal ion dopants in quantum-sized TiO2: correlation between photoreactivity and charge carrier recombination dynamics. J Phys Chem 98:13669–13679
Choi J, Park H, Hoffmann MR (2010) Effects of single metal-ion doping on the visible-light photoreactivity of TiO2. J Phys Chem C 114:783–792
Chung J, Chung JW, Kwak S-Y (2015) Adsorption-assisted photocatalytic activity of nitrogen and sulfur codoped TiO2 under visible light irradiation. Phys Chem Chem Phys 17:17279–17287
Cullity BD, Graham CD (2009) Introduction to magnetic materials, 2nd edn. Wiley, Hoboken
Dejene FB, Onani MO, Tarus PK (2016) The effect of rate of hydrolysis on structural and optical properties of the TiO2 nanoparticles prepared by a sol-gel method. Phys B 480:213–218
Devi LG, Kottam N, Murthy BN, Kumar SG (2010) Enhanced photocatalytic activity of transition metal ions Mn2+, Ni2+ and Zn2+ doped polycrystalline titania for the degradation of aniline blue under UV/solar light. J Mol Catal A: Chem 328:44–52
Farzadkia M, Bazrafshan E, Esrafili A, Yang J-K, Shirzad-Siboni M (2015) Photocatalytic degradation of metronidazole with illuminated TiO2 nanoparticles. J Environ Health Sci Eng 13:35
Ganesh I, Gupta AK, Kumar PP, Sekhar PCS, Radha K, Padmanabham G, Sundararajan G (2012) Preparation and characterization of Ni-doped TiO2 materials for photocurrent and photocatalytic applications. Sci World J 2012:1–16
Gomathi Devi L, Narasimha Murthy B (2008) Characterization of Mo Doped TiO2 and its enhanced photo catalytic activity under visible light. Catal Lett 125:320–330
Jiang W, Joens JA, Dionysiou DD, O’Shea KE (2013) Optimization of photocatalytic performance of TiO2 coated glass microspheres using response surface methodology and the application for degradation of dimethyl phthalate. J Photochem Photobio A Chem 262:7–13
Jin Y, Zhao G, Wu M, Lei Y, Li M, Jin X (2011) In situ induced visible-light photoeletrocatalytic activity from molecular oxygen on carbon aerogel-supported TiO2. J Phys Chem C 115:9917–9925
Jing Y, Li L, Zhang Q, Lu P, Liu P, Lü X (2011) Photocatalytic ozonation of dimethyl phthalate with TiO2 prepared by a hydrothermal method. J Hazard Mater 189:40–47
Kaur R, Thakur S, Singh K (2014) Effect of two different sites substitution on structural and optical properties of Bi4V2O11 − δ. Phys B 440:78–82
Khan H, Swati IK (2016) Fe3+-doped anatase TiO2 with dd transition, oxygen vacancies and Ti3+ centres: synthesis, characterization, UV/vis photocatalytic and mechanistic studies. Ind Eng Chem Res 55:6619–6633
Kim EY, Kim DS, Ahn BT (2009) Synthesis of mesoporous TiO2 and its application to photocatalytic activation of methylene blue and E. coli. Bull Korean Chem Soc 30:193–196
Lahl N, Singh Singheiser L, Hilpert K, Bahadur D (2000) Crystallisation kinetics in AO–Al2O3–SiO2–B2O3 glasses (A = Ba, Ca, Mg). J Mater Sci 35:3089–3096
Luttrell T, Halpegamage S, Tao J, Kramer A, Sutter E, Batzill M (2014) Why is anatase a better photocatalyst than rutile?-Model studies on epitaxial TiO2 films. Sci Rep 4:1–7
Nakata K, Fujishima A, Photoch J (2012) TiO2 photocatalysis: design and applications. C Photochem Rev 13:169–189
Ni M, Leung MKH, Leung DYC, Sumathy K (2007) A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew Sustain Energy Rev 11:401–425
Niyaz AM, Haque MM, Khan A, Muneer M, Vijayalakshmi S (2014) Photocatalytic degradation of herbicide Bentazone in aqueous suspension of TiO2: mineralization, identification of intermediates and reaction pathways. Environ Technol 35:407–415
Oliveira TF, Chedeville O, Cagnon B, Fauduet H (2011) Degradation kinetics of DEP in water by ozone/activated carbon process: influence of pH. Desalination 269:271–275
Razali MH, Fauzi MNA, Mohamed AR, Sreekantan S (2013) Morphological, structural and optical properties study of transition metal ions doped TiO2 nanotubes prepared by hydrothermal method. Int J Mater Mech Manuf 1:314–318
Razali MH, Noor AFM, Yusoff M (2015) Co2+ doped TiO2 nanotubes visible light photocatalyst synthesized by hydrothermal method for methyl orange degradation. Int Res J Eng Technol 2:92–96
Roy P, Kim D, Lee K, Spiecker E, Schmuki P (2010) TiO2 nanotubes and their application in dye-sensitized solar cells. Nano Scale 2:45–59
Sabry RS, Hyidarie YKA, Kudhier MA (2016) Synthesis and photocatalytic activity of TiO2 nanoparticles prepared by sol–gel method. J Sol–Gel Sci Technol 78:299–306
Singla P, Pandey OP, Singh K (2016) Study of photocatalytic degradation of environmentally harmful phthalate esters using Ni-doped TiO2 nanoparticles. Int J Environ Sci Technol 13:849–856
Teixeira S, Martins PM, Lanceros-Mendez S, Kuhn K (2016) Reusability of photocatalytic TiO2 and ZnO nanoparticles immobilized in poly(vinylidene difluoride)-co-trifluoroethylene. Appl Surf Sci 384:497–504
Tripathi AK, Singh MK, Mathpal MC, Mishra SK, Agarwal A (2013) Study of structural transformation in TiO2 nanoparticles and its optical properties. J Alloy Compd 549:114–120
Umar K, Haque MM, Muneer M, Harada T, Matsumura M (2013) Mn and La doped TiO2: synthesis, characterization and photocatalytic activity for the decolourization of three different chromophoric dyes. J Alloy Compd 578:431–438
Wang B, Wang W, Li Y, Zhai J (2011) Preparation and characterization of Fe3+-doped TiO2 on fly ash cenospheres for photocatalytic application. Appl Surf Sci 257:3473–3479
Wei X, Zhu G, Fang J, Chen J (2013) Characterization, and photocatalysis of well-dispersible phase-pure anatase TiO2 nanoparticles. Int J Photoenergy 2013:1–6
Wu JCS, Chen CH (2004) A visible-light response vanadium-doped titania nanocatalyst by sol–gel method. J Photochem Photobiol, A 163:509–515
Yu J, Xiang Q, Zhou M (2009) Preparation, characterization and visible-light-driven photocatalytic activity of Fe-doped titania nanorods and first-principles study for electronic structures. Appl Catal B 90:595–602
Zhang D (2012) Chemical synthesis of Ni/TiO2 nanophotocatalyst for UV/visible light assisted degradation of organic dye in aqueous solution. J Sol–Gel Sci Technol 58:312–318
Acknowledgement
Authors are very thankful to Dr. Satwinder Singh Danewalia and Ms. Neetu Bansal for their help and discussions during the present work. The work is supported financially by Thapar University, Patiala.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: Necip Atar.
Rights and permissions
About this article
Cite this article
Kaur, R., Singla, P. & Singh, K. Transition metals (Mn, Ni, Co) doping in TiO2 nanoparticles and their effect on degradation of diethyl phthalate. Int. J. Environ. Sci. Technol. 15, 2359–2368 (2018). https://doi.org/10.1007/s13762-017-1573-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-017-1573-y