Abstract
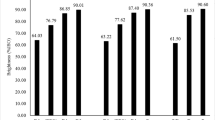
The aim of this work was to study the application of two biodegradable chelating agents, pyridine-2,6-dicarboxylic acid (PDA) and methylglycinediacetic acid (MGDA), in the treatment of the pulp, prior to hydrogen peroxide bleaching. Such compounds must remove transition metals (Mn, Fe and Cu) from pulp, that catalyze the degradation of hydrogen peroxide, and Ca, which is also problematic due to the formation of precipitates that accumulate in the equipment. Computer simulations were first performed to study the best conditions for metal complexation, and optimum pH was defined as 5–5.5 for PDA and 6.5–7 for MGDA. Metals removal from the pulp, as well as the subsequent bleaching process (Q-P1-Paa-P2), were tested experimentally, and performances were compared to ethylenediaminetetracetic acid (EDTA). PDA removed both Mn and Ca efficiently, leaving most Mg in the pulp after first chelation stage, while MGDA had a lower Ca removal, even using a higher pH and concentration. Residual hydrogen peroxide and kappa number after peroxide stages showed a similar bleaching efficiency between the studied compounds and EDTA.




Similar content being viewed by others
References
Abbot J, Brown DG (1990) Stabilization of iron-catalyzed hydrogen-peroxide decomposition by magnesium. Can J Chem 68:1537–1543
Area MC, Felissia FE (2005) Chelating agents management to obtain TCF bleached Eucalyptus grandis kraft pulps. Appita J 58:143–148
Bajpai P (2012) Environmentally benign approaches for pulp bleaching, 2nd edn. Elsevier, Amsterdam
BASF (2007) Technical information Ti/EVD 1418 e—Trilon® M types
Black G, Depp E, Corson BB (1949) Oxidation of certain methylpyridines to pyridine carboxylic acids. J Org Chem 14:14–21
Boskamp JV (1990) Detergent composition. Patent number EP358472
Bucheli-Witschel M, Egli T (2001) Environmental fate and microbial degradation of aminopolycarboxylic acids. FEMS Microbiol Rev 25:69–106
Chandraghargi RS (2003) Interaction of manganese and copper with wood pulp fibers. Thesis for Master of Applied Science, Department of Chemical and Biological Engineering, University of British Columbia, Vancouver, Canada
Colodette JL, Rothenberg S, Dence CW (1989) Factors affecting hydrogen-peroxide stability in the brightening of mechanical and chemimechanical pulps. II. Hydrogen-peroxide stability in the presence of sodium-silicate. J Pulp Pap Sci 15:3–10
De Almeida JMH, Bachus H, Djodikromo ZP, Doerfler C, Hage R, Lienke J (2008) Bleaching of substrates. Patent number WO2008086937
Dournel P (2009) Process for the bleaching of paper pulp. Patent number WO2009144190
Elsander A, Ek M, Gellerstedt G (2000) Oxalic acid formation during ECF and TCF bleaching of kraft pulp. Tappi J 83:73–77
Hong APK, Chen TC (1996) Chelating extraction and recovery of cadmium from soil using pyridine-2,6-dicarboxylic acid. Water Air Soil Pollut 86:335–346
Hyvonen H, Orama M, Arvelac R, Henriksson K, Saarinen H, Aksela R, Parene A, Jakara J, Renvall I (2006) Studies on three new environmentally friendly chelating ligands. Appita J 59:142–149
Jefferis J, Zack K (2011) Detergent composition. Patent number WO2011100344
Jones PW, Williams DR (2001) Speciation efficiency indices (SEI) and readily-biodegradable indices (RBI) for optimising ligand control of environmental and associated industrial processes. Int J Environ Anal Chem 81:73–88
Jones PW, Williams DR (2002) Chemical speciation simulation used to assess the efficiency of environment-friendly EDTA alternatives for use in the pulp and paper industry. Inorg Chim Acta 339:41–50
Knepper TP (2003) Synthetic chelating agents and compounds exhibiting complexing properties in the aquatic environment. Trac Trend Anal Chem 22:708–724
Kujala M, Sillanpaa M, Ramo J (2004) A method to leach manganese and some other metal cations from pulp matrix to aqueous phase for the subsequent ICP-AES analysis: a potential tool for controlling the metal profile in a pulp bleaching process. J Clean Prod 12:707–712
Lapierre L, Berry R, Bouchard J (2003) The effect of magnesium ions and chelants on peroxide bleaching. Holzforschung 57:627–633
List TC, Reinbold RS (2010) Compositions and methods for removing scale and inhibiting formation thereof. Patent number US2010000579
Loureiro PEG, Evtuguin DV, Carvalho MGVS (2011) The final bleaching of eucalypt kraft pulps with hydrogen peroxide: relationship with industrial ECF bleaching history and cellulose degradation. J Chem Technol Biotechnol 86:381–390
Macauley E, Hong A (1995) Chelation extraction of lead from soil using pyridine-2,6-dicarboxylic acid. J Hazard Mater 40:257–270
Martell AE, Smith RM (2004) NIST standard reference database 46 version 8.0. NIST critically selected stability constants of metal complexes database. US Department of Commerce, National Institute of Standards and Technology
Martins JG, Neto I, Pinto ISS, Soares EV, Barros MT, Soares HMVM (2014) Alternative chelating agents: evaluation of the ready-biodegradability and complexation properties. J Environ Sci Health A 49:1–11
Metsarinne S, Ronkainen E, Tuhkanen T, Aksela R, Sillanpaa M (2007) Biodegradation of novel amino acid derivatives suitable for complexing agents in pulp bleaching applications. Sci Total Environ 377:45–51
Ni Y, Liu Z (2000) Pulp bleaching. In: Kirk RE, Othmer DF (eds) Encyclopedia of chemical technology. Wiley, New York
OECD (1992) Ready biodegradability 301. OECD guideline for testing of chemicals, vol 301
Potucek F, Milichovsky M (2000) Kraft pulp bleaching with hydrogen peroxide and peracetic acid. Chem Pap 54:406–411
Povoas TM, Angelico DAG, Egas APV, Loureiro PEG, Gando-Ferreira LM, Carvalho MGVS (2012) Prebleaching of eucalypt kraft pulp with OP stages: effect of an acid pretreatment or chelation step. Tappi J 11:31–38
Rattinger GB, Cotter B, Fair MJ (1994) Liquid automatic dishwashing composition. Patent number WO9405763
Renvall I, Askela R, Paren A (1997) Process for bleaching of a high yield pulp. Patent number WO9730209
Rudie A (2000) Calcium in pulping and bleaching. Tappi J 83:36–37
Schecher WD, McAvoy DC (2003) MINEQL+ a chemical equilibrium modeling system, version 4.5 for Windows. User’s Manual Hallowell, Maine
Seccombe R (2008) Process for the bleaching of paper pulp. Patent number WO2008101952
Seccombe R, Dournel P (2007) Process for the bleaching of mechanical paper pulp. Patent number WO2007085579
Sillanpaa M, Raimo KB, Sihvonen ML (1995) Determination of Edta and Dtpa as their Fe(III) complexes in pulp and paper-mill process and waste-waters by liquid-chromatography. Anal Chim Acta 303:187–192
Singer AW, McElvain SM (1955) Organic synthesis. Collective 3:740
Turner ET, Moore SB, Leuck JF (1997) Bleaching composition. Patent number US5616280
Wuorimaa A, Jokela R, Aksela R (2006) Recent developments in the stabilization of hydrogen peroxide bleaching of pulps: an overview. Nord Pulp Pap Res J 21:435–443
Xiao HB, Tao XM, Wang YC, Qian SX, Shi GH, Li H (2008) Dipicolinate as acceptor in D–π–A chromophores: synthesis, characterization and fluorescence following single- and two-photon excitation. Tetrahedron Lett 49:6819–6822
Acknowledgments
This work was financially supported by FEDER funds through the Programa Operacional Factores de Competitividade—COMPETE and national funds by FCT—Fundação para a Ciência e Tecnologia within the Project PTDC-AAC-AMB-111206-2009.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pinto, I.S.S., Ascenso, O.S., Barros, M.T. et al. Pre-treatment of the paper pulp in the bleaching process using biodegradable chelating agents. Int. J. Environ. Sci. Technol. 12, 975–982 (2015). https://doi.org/10.1007/s13762-013-0480-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-013-0480-0