Abstract
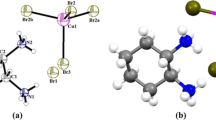
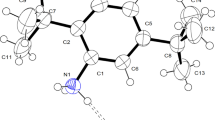
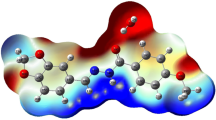
The new organic salt m-xylylenediaminium bis (tetrafluoroborate) monohydrate was prepared from a mixture of an aqueous solution of tetrafluoroborate acid and m-xylylenediamine. Crystals were grown by slow evaporation method and characterized by single-crystal X-ray diffraction. It crystallizes in the monoclinic system, space group P21/c, with a = 5.4839(2), b = 10.9453(3), c = 23.0760(6) Å, β = 95.105(1)°, V = 1379.59(14) Å3 and Z = 4. The intermolecular interactions were identified by Hirshfeld Surfaces analysis and their associated 2D fingerprint plots. Photoluminescence and UV–Vis absorption were carried out to identify optical properties and determine the energy gap. Dielectric and conduction mechanism were studied at different frequencies, in the temperature range of 303–390 K. A comparison of the optimized structure at the B3LYP/6-311++G(2d,2p) level, using the density functional theory, was made with the experimentally determined structure. Comprehensive experimental and theoretical structural studies were carried out by FT-IR. Molecular electrostatic potentials were also studied and discussed.
Graphic abstract













Similar content being viewed by others
References
M.R.B.M. Rejab, M.H.B.M. Hamdan, M. Quanjin, J.P. Siregar, D. Bachtiar, Y. Muchlis, Historical development of hybrid materials. Mat. Sci. Mat. Eng. 322, 1516–1520 (2019)
H. Zhang, M. Fallahi, Electro-optic waveguide based on hybrid sol–gel doped with organic chromophore. Optcom. 248, 415 (2005)
P. Mayuri, P. Nellepalli, K. Vijayakrishna, A.S. Kumar, Tuning poly(ionic liquid) as a facile anion (hexacyanoferrate(III) ion)-exchanger after being adsorbed on graphitic nano material and its versatile electrocatalytic oxidation of ascorbic acid. J. Phys. Chem. C 123(32), 19637 (2019)
A. Wiscons, M. Zeller, J.L.C. Rowsell, Anion exchange in cationic frameworks: structures of channel-forming triarylpyrylium tetrafluoroborate salts. Cryst. Growth Des. 16, 4 (2016)
M. Enayati, H. Faghihian, N-butyl-pyridinium tetrafluoroborate as a highly efficient ionic liquid for removal of dibenzothiophene from organic solutions. J. Fuel Chem. Technol. 43, 195 (2015)
M.D. Zidan, M.M. Al-Ktaifani, A. Allahham, Nonlinear optical investigation of the Tris(2′,2-bipyridyl)iron(II) tetrafluoroborate using z-scan technique. Opt. Laser Technol. 90, 174 (2017)
N.O. Alzamil, G.M. Al-Enzi, A.H.A. Crystalsri, I. Abdi, A. Abdi, Synthesis, crystal structures and characterization of two nonmetal cation tetrafluoroborates. Crystals 10(9), 812 (2020)
M. Ehtisham, F. Wani, I. Wani, P. Kaur, S. Nissar, Fundamentals of in situ hybridization: a review. Int. Res. J. Clin. Med. 1, 23 (2016)
C.E. Carraher, M.R. Roner, K. Shahi, G. Barot, Structural consideration in designing organotin polyethers to arrest the growth of breast cancer cells in vitro. Materials 4(4), 801 (2010)
S.A. Dharaskar, K.L. Wasewar, M.N. Varma, D.Z. Shende, C.K. Yoo, Synthesis, characterization and application of 1-butyl-3-methylimidazolium tetrafluoroborate for extractive desulfurization of liquid fuel. Arab. J. Chem. 9(4), 578 (2016)
Z.Y. Yu, J. Zhang, S.S. Liu, Biochemical and gene expression effects of 1-alkyl-3-methylimidazolium tetrafluoroborate on Vibrio qinghaiensis sp.-Q67. J. Hazard. Mater. 300, 483 (2015)
V. Murugesan, M. Saravanabhavan, A. Chandramohan, G. Raja, M. Sekar, Pharmacological investigation of 2-aminobenzothiazolium-4-methylbenzenesulphonate: synthesis, spectral characterization and structural elucidation. J. Photochem. Photobiol. B: Biol. 151, 248 (2015)
V. Murugesan, M. Saravanabhavan, Synthesis, spectral, structural characterization and biological investigation of m-xylylenediaminium-bis (p-toluenesulfonate) monohydrate. J. Photochem. Photobial. B148, 358 (2015)
T. Umemoto, M.N. Nagayoshi, N′-difluoro-1,4-diazoniabicyclo[2.2.2]octane salts, highly reactive and easy-to-handle electrophilic fluorinating agents. Bull. Chem. Soc. Jpn. 69, 2287 (1996)
K.K. Laali, G.I. Borodkin, First application of ionic liquids in electrophilic fluorination of arenes SelectfluorTM (F-TEDA-BF4) for “green” fluorination. J. Chem. Soc., Perkin Trans. 2, 953 (2002)
S. Budavari, in The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals, ed. by N.J. Rahway (Merck, USA, 1989)
J. Chang, S.W. Kang, CO2 separation through poly (vinylidene fluoride-co-hexafluoropropylene) membrane by selective ion channel formed by tetrafluoroboric acid. Chem. Eng. J. 306, 1189 (2016)
O.V. Dolomanov, L.J. Bourhis, R.J. Gildea, J.A.K. Howard, H. Puschmann, OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 42(2), 339 (2009)
G.M. Sheldrick, Integrated space-group and crystal-structure determination. Acta Cryst. A71, 3 (2015)
G.M. Sheldrick, Crystal structure refinement with SHELXL. Acta Cryst. C71, 3 (2015)
K. Brandenburg, DIAMOND—visual crystal structure information system. J. Appl. Crystallogr. 32, 1028 (1999)
C.F. Macrae, I.J. Bruno, J.A. Chisholm, P.R. Edgington, P. McCabe, E. Pidcock, L. Rodriguez-Monge, R. Taylor, J.V.D. Streek, P.A. Wood, Mercury CSD 2.0—new features for the visualization and investigation of crystal structures. J. Appl. Cryst. 41, 466 (2008)
S.K. Wolff, D.J. Grimwood, J.J. McKinnon, M.J. Turner, D. Jayatilaka, M.A. Spackman, CrystalExplorer (Version 3.1) (University of Western Australia, 2012)
J. Heyd, G.E. Scuseria, Efficient hybrid density functional calculations in solids: assessment of the Heyd–Scuseria–Ernzerhof screened Coulomb hybrid functional. J. Chem. Phys. 121(3), 1187 (2004)
S. Chaouachi, S. Elleuch, B. Hamdi, R. Zouari, Experimental (FTIR, Raman, UV–visible and PL) and theoretical (DFT and TDDFT) studies on bis(8-hydroxyquinolinium) tetrachlorocobaltate(II) compound. J. Mol. Struct. 1125(Ii), 149 (2016)
C. Lee, W. Yang, R.G. Parr, Development of the Colic-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B37, 785 (1988)
Y. Arfaoui, M.L. Efrit, Besbes, , N Theoretical investigations on the mechanistic pathway of the thermal rearrangement of substituted N-acyl-2,2-dimethylaziridines. J. Mol. Mod. 19(10), 4603 (2013)
M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J.R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G.A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H.P. Hratchian, A.F. Izmaylov, J. Bloino, G. Zheng, J.L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J.A. Montgomery, J.E. Peralta, F. Ogliaro, M. Bearpark, J.J. Heyd, E. Brothers, K.N. Kudin, V.N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J.C. Burant, S.S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J.M. Millam, M. Klene, J.E. Knox, J.B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R.E. Stratmann, O. Yazyev, A.J. Austin, R. Cammi, C. Pomelli, J.W. Ochterski, R.L. Martin, K. Morokuma, V.G. Zakrzewski, G.A. Voth, P. Salvador, J.J. Dannenberg, S. Dapprich, A.D. Daniels, O. Farkas, J.B. Foresman, J.V. Ortiz, J. Cioslowski, D.J. Fox, Gaussian 09, Revision A.1 (Gaussian Inc., Wallingford, 2009).
R.D. Dennington, T.A. Keith, J.M. Millam, GaussView 5.0.8 (Gaussian Inc., Wallingford, 2008).
S. Hassen, H. Chebbi, M.F. Zid, Y. Arfaoui, Crystal structure, spectroscopic study, photoluminescent properties and DFT calculations of the 2-guanidinobenzimidazolium dichloride and dibromide monohydrate salts. J. Mol. Struct. 1167, 1 (2018)
N. Sundaraganesan, B.D. Joshua, K. Settu, Vibrational spectra and assignments of 5-amino-2-chlorobenzoic acid by ab initio Hartree-Fock and density functional methods. Spectrochim. Acta A: Mol. Biomol. Spectrosc. 66, 381 (2007)
M. Hemamalini, P.T. Muthiah, J. DE Muthiah, Hydrogen-bonding patterns in trimethoprim tetra¬fluoroborate. Acta Crystallogr. E61, o4107 (2005)
K. Balabsubramani, P.T. Muthiah, J.D.E. Lynch, Hydrogen-bonding patterns in pyrimethamine tetrafluoroborate. Acta Crystallogr. E63, o2966 (2007)
W.H. Baur, The geometry of polyhedral distortions. Predictive relationships for the phosphate group. Acta Crystallogr. B30, 1191 (1974)
A. Guesmi, T. Roisnel, H. Marouani, Featuring non-covalent interactions in m-xylylenediaminium bis(perchlorate) monohydrate: synthesis, characterization and Hirshfeld surface analysis. J. Mol. Struct. 1194, 66 (2019)
V.V. Ghazaryan, M. Fleck, A.M. Petrosyan, Sarcosine sarcosinium tetrafluoroborate and sarcosine sarcosinium perchlorate: synthesis, structure and vibrational spectra. J. Mol. Struct. 1021, 130 (2012)
R.P. Sharma, R. Bala, R. Sharma, P. Venugopalan, J.M. Salas, M. Quiros, Second sphere coordination in anion binding: synthesis, spectroscopic characterization and single crystal X-ray structure determination of hexaamminecobalt(III) dichloride tetrafluoroborate. J. Mol. Struct. 794, 341 (2006)
A. Guesmi, S. Gatfaoui, T. Roisnel, H. Marouani, M-xylylenediaminium sulfate: crystal structure and Hirshfeld surface analysis. Acta Cryst. E72, 776 (2016)
J. Tauc (ed.), Aamorphous and Liquid Semiconductor (Plenum Press, New York, 1979)
B.D. Viezbicke, S. Patel, B.E. Davis, D.P. Birnie, Evaluation of the Tauc method for optical absorption edge determination: ZnO thin films as a model system. Phys. Status Solidi (B) 252(8), 1700 (2015)
X. Ziang, L. Shifeng, Q. Laixiang, P. Shuping, W. Wei, Y. Yu, G.G. Qin, Refractive index and extinction coefficient of CH3NH3PbI3 studied by spectroscopic ellipsometry. Opt. Mater. Express 5(1), 29 (2015)
S. Nandhini, K. Sudhakar, S. Muniyappan, P. Murugakoothan, Systematic discussions on structural, optical, mechanical, electrical and its application to NLO devices of a novel semi-organic single crystal : guanidinium tetrafluoroborate (GFB). Opt. Laser Technol. 105, 249 (2018)
Z.S. Petrovic, I. Javni, A. Waddon, G. Banhegyi, Structure and properties of polyurethane–silica nanocomposites. J. Appl. Poly. Sci. 76, 133 (1999)
Z. Ouerghi, H. Gornitzka, E. Temel, I. Dridi, R. Kefi, A new non-centrosymmetric Chlorobismuthate(III) hybrid material: crystal structure, optical properties and antibacterial study. J. Mol. Struct. 1181, 338 (2019)
I. Madhi, M. Saadoun, B. Bessaïs, Sensing characteristics of zinc oxide thin films deposited by spray pyrolysis onto tubular Pyrex substrate. Mater. Sci. Semi. Proc. 16(6), 1365 (2013)
H. Mahamoud, B. Louati, F. Hlel, K. Guidara, Impedance and modulus analysis of the (Na0.6Ag0.4)2PbP2O7 compound. J. All. Comp. 509(20), 6083 (2011)
C.K. Suman, K. Prasad, R.N.P. Choudhary, Complex impedance studies on tungsten-bronze electroceramic: Pb 2Bi3LaTi5O18. J. Mater. Sci. 41(2), 369 (2006)
Z. Ouerghi, T. Roisnel, R. Fezai, R. Kefi, Physico-chemical characterization, Hirshfeld surface analysis and opto-electric properties of a new hybrid material: tris (2-amino-5-chloropyridinium) hexachlorobismuthate(III). J. Mol. Struct. 1173, 439 (2018)
P.P. Sahay, R.K. Mishra, S.N. Mishra, S. Jha, M. Shamsuddin, AC transport properties of nanocrystalline SnO2 semiconductor. Ceram. Int. 38(2), 1281 (2012)
R. Mbarki, A. Mnif, A.H. Hamzaoui, Structural, dielectric relaxation and electrical conductivity behavior in MgO powders synthesized by sol-gel. Mat. Sci. Semi. Proc. 29, 300 (2015)
M.S. Masoud, S.A. El-Enein, A.M. Ramadan, A.S. Goher, Thermal properties of some biologically active 5-(p-substituted phenylazo)-6-aminouracil complexes. J. Anal. Appl. Pyrol. 81(1), 45 (2008)
Acknowledgements
This work is supported by the Tunisian National Ministry of Higher Education and Scientific Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Haddaoui, S., Ouerghi, Z., Arfaoui, Y. et al. Structural characterization, dielectric properties, optical and theoretical DFT study of (C8H14N2)(BF4)2·H2O compound. J IRAN CHEM SOC 18, 2065–2077 (2021). https://doi.org/10.1007/s13738-021-02165-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-021-02165-4