Abstract
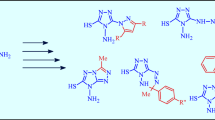
A novel, simple, and efficient protocol is described for the synthesis of 1-alkyl-2-(aryloxy)methyl-1H-pyrrolo[2,3-b]quinoxalines via the one-pot reaction of phenol or 2-naphthol derivatives, propargyl bromide, and N-alkyl-3-chloroquinoxaline-2-amines in the presence of palladium catalyst. This one-pot reaction is carried out in the absence of any copper salt at room temperature. The reaction product is dependent on the nature of the base used. The use of morpholine, as the base, affords the final cyclized product, and triethylamine only gives the coupling product.
Graphical abstract





Similar content being viewed by others
Change history
26 September 2018
The original version of this article unfortunately contained mistakes. Table 2, entry 6. The structure of the product 4f is missing.
References
J. Harmenberg, A. Åkesson-Johansson, A. Gräslund, T. Malmfors, J. Bergman, B. Wahren, S. Åkerfeldt, L. Lundblad, S. Cox, Antiviral Res. 15, 193 (1991)
R. David, Expert Opin. Investig. Drugs 7, 1063 (1998)
M. Naylor, M. Stephens, J. Nolan, B. Sutton, J. Tocher, E. Fielden, G. Adams, I. Stratford, Anticancer Drug Des. 8, 439 (1993)
G. Campiani, E. Morelli, S. Gemma, V. Nacci, S. Butini, M. Hamon, E. Novellino, G. Greco, A. Cagnotto, M. Goegan, J. Med. Chem. 42, 4362 (1999)
G. Campiani, F. Aiello, M. Fabbrini, E. Morelli, A. Ramunno, S. Armaroli, V. Nacci, A. Garofalo, G. Greco, E. Novellino, J. Med. Chem. 44, 305 (2001)
J. Guillon, S. Moreau, E. Mouray, V. Sinou, I. Forfar, S.B. Fabre, V. Desplat, P. Millet, D. Parzy, C. Jarry, Biorg. Med. Chem. 16, 9133 (2008)
G. Szabó, R. Kiss, D. Páyer-Lengyel, K. Vukics, J. Szikra, A. Baki, L. Molnár, J. Fischer, G.M. Keserű, Bioorg. Med. Chem. Lett. 19, 3471 (2009)
V. Desplat, S. Moreau, A. Gay, S.B. Fabre, D. Thiolat, S. Massip, G. Macky, F. Godde, D. Mossalayi, C. Jarry, J. Enzyme Inhib. Med. Chem. 25, 204 (2010)
B. Prasad, K.S. Kumar, P.V. Babu, K. Anusha, D. Rambabu, A. Kandale, G. Vanaja, A.M. Kalle, M. Pal, Tetrahedron Lett. 53, 6059 (2012)
A. Nakhi, M.S. Rahman, R. Kishore, C.L.T. Meda, G.S. Deora, K.V. Parsa, M. Pal, Bioorg. Med. Chem. Lett. 22, 6433 (2012)
J. Miyashiro, K.W. Woods, C.H. Park, X. Liu, Y. Shi, E.F. Johnson, J.J. Bouska, A.M. Olson, Y. Luo, E.H. Fry, Bioorg. Med. Chem. Lett. 19, 4050 (2009)
V. Desplat, A. Geneste, M.-A. Begorre, S.B. Fabre, S. Brajot, S. Massip, D. Thiolat, D. Mossalayi, C. Jarry, J. Guillon, J. Enzyme Inhib. Med. Chem. 23, 648 (2008)
J.J. Li, J. Org. Chem. 64, 8425 (1999)
D.E. Ames, M.I. Brohi, J. Chem. Soc. Perkin Trans. 1, 1384 (1980)
A. Arcadi, S. Cacchi, G. Fabrizi, L.M. Parisi, Tetrahedron Lett. 45, 2431 (2004)
D.M. D’Souza, T.J. Mueller, Chem. Soc. Rev. 36, 1095 (2007)
K. Sonogashira, Y. Tohda, N. Hagihara, Tetrahedron Lett. 16, 4467 (1975)
R. Chinchilla, C. Najera, Chem. Rev. 107, 874 (2007)
S. Ma, E. Negishi, J. Am. Chem. Soc. 117, 6345 (1995)
C. Chowdhury, G. Chaudhuri, S. Guha, A. k. Mukherjee, N.G. Kundu, J. Org. Chem. 63, 1863 (1998)
B. Kundu, B. Nandi, J. Org. Chem. 66, 4563 (2001)
M.M. Heravi, S. Sadjadi, Tetrahedron 65, 7761 (2009)
A. Keivanloo, M. Bakherad, A. Rahimi, S.A.N. Taheri, Tetrahedron Lett. 51, 2409 (2010)
A. Keivanloo, M. Bakherad, A. Rahimi, Synthesis 2010, 1599, (2010)
A. Keivanloo, M. Bakherad, M. Rahmani, A. Rahimi, Monatsh. Chem. 144, 859 (2013)
A. Keivanloo, S.S. Kazemi, H. Nasr-Isfahani, A. Bamoniri, Tetrahedron 72, 6536 (2016)
S.S. Kazemi, A. Keivanloo, H. Nasr-Isfahani, A. Bamoniri, RSC Adv. 6, 92663 (2016)
T. Besharati-Seidani, A. Keivanloo, B. Kaboudin, T. Yokomatsu, RSC Adv. 6, 83901 (2016)
A. Elangovan, Y.-H. Wang, T.-I. Ho, Org. Lett. 5, 1841 (2003)
P. Siemsen, R.C. Livingston, F. Diederich, Angew Chem. Int. Ed. 39, 2632 (2000)
J. Cheng, Y. Sun, F. Wang, M. Guo, J.-H. Xu, Y. Pan, Z. Zhang, J. Org. Chem. 69, 5428 (2004)
H. Zhong, J. Wang, L. Li, R. Wang, Dalton Trans. 43, 2098, (2014)
N.E. Leadbeater, B.J. Tominack, Tetrahedron Lett. 44, 8653 (2003)
Acknowledgements
We gratefully acknowledge the financial support of the Research Council of the Shahrood University of Technology.
Author information
Authors and Affiliations
Corresponding author
Additional information
The original version of this article was revised: The structure of the product 4f is shown in Table 2, entry 6.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Keivanloo, A., Fakharian, M., Nabid, M.R. et al. Novel one-pot synthesis of 1-alkyl-2-(aryloxy)methyl-1H-pyrrolo[2,3-b]quinoxalines via copper-free Sonogashira coupling reaction. J IRAN CHEM SOC 16, 151–160 (2019). https://doi.org/10.1007/s13738-018-1492-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-018-1492-y