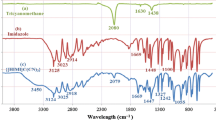
Abstract
1,4-Dinitropyrazine-1,4-diium trinitromethanide {[1,4-pyrazine-NO2][C(NO2)3]2} as a novel nanostructured molten salt (NMS) catalyzed the synthesis of 2-amino-3,5-dicarbonitrile-6-sulfanylpyridine derivatives via the one-pot three-component condensation reaction between several aromatic aldehyde, malononitrile and benzyl mercaptan at room temperature under solvent-free conditions. The synthesized NMS catalyst was fully characterized by FTIR, 1H NMR, 13CNMR, mass, thermal gravimetric, X-ray diffraction patterns, scanning electron microscopy and transmission electron microscopy analysis. The major advantages of described methodology are mildness, ease of separation, good yields and short reaction times. A rational mechanism was suggested for the final step of the 2-amino-3,5-dicarbonitrile-6-sulfanylpyridines synthesis. We think that the proposed mechanism has potential for entering into the graduate text book in the future.












Similar content being viewed by others
References
J.M. Berg, J.L. Tymoczko, L. Stryer, Biochemistry (W.H. Freeman and Company, New York, 2002)
G. Hamasaka, H. Tsuji, Y. Uozumi, Synlett 26, 2037 (2015)
J.A. Joule, K.M. Mills, Heterocyclic Chemistry, 5th edn. (Wiley, London, 2010)
J.M. Quintela, C. Peinador, L.M. Botana, M. Estèvez, R. Riguera, Bioorg. Med. Chem. 5, 1543 (1997)
J.M. Quintela, C. Peinador, M.C. Veiga, L.M. Botana, A. Alfonso, R. Riguera, Eur. J. Med. Chem. 33, 887 (1998)
J.M. Quintela, C. Peinador, Tetrahedron 52, 10497 (1996)
A.M.E. Attia, A.E.H.A.A. Ismail, Tetrahedron 59, 1749 (2003)
U.V. Desai, M.A. Kulkarni, K.S. Pandit, A.M. Kulkarni, P.P. Wadgaonkar, Green Chem. Lett. Rev. 7, 228 (2014)
P.V. Shinde, V.B. Labade, B.B. Shingate, M.S. Shingare, J. Mol. Catal. A: Chem. 336, 100 (2011)
J. Safaei-Ghomi, M.A. Ghasemzadeha, M. Mehrabi, Sci. Iran. C 20, 549 (2013)
J. Safaei-Ghomi, M.A. Ghasemzadeh, J. Sulfur Chem. 34, 233 (2013)
N.M. Evdokimov, I.V. Magedov, A.S. Kireev, A. Kornienko, Org. Lett. 8, 899 (2006)
N.M. Evdokimov, A.S. Kireev, A.A. Yakovenko, M.Y. Antipin, I.V. Magedov, A. Kornienko, J. Org. Chem. 72, 3443 (2007)
J. Safaei-Ghomi, H. Shahbazi-Alavi, E. Heidari-Baghbahadorani, RSC Adv. 4, 50668 (2014)
A. Mola, S. Hussain, RSC Adv. 4, 29750 (2014)
S. Banerjee, G. Sereda, Tetrahedron Lett. 50, 6959 (2009)
T.L. Greaves, C.J. Drummond, Chem. Rev. 108, 206 (2008)
M. Freemantle, Chem. Eng. News 76, 32 (1998)
J.H. Davis, Chem. Lett. 33, 1072 (2004)
R. Hayes, G.G. Warr, R. Atkin, Chem. Rev. 115, 6357 (2015)
Y. Huang, H. Gao, B. Twamley, J.M. Shreeve, Eur. J. Inorg. Chem. 14, 2025 (2007)
J.T. Wu, J.G. Zhang, X. Yin, Z.Y. Cheng, C.X. Xu, New J. Chem. 39, 5265 (2015)
A.J. Kirby, The Anomeric Effect and Related Stereoelectronic Effects at Oxygen (Springer, Berlin, 1983)
M.A. Zolfigol, F. Afsharnadery, S. Baghery, S. Salehzadeh, F. Maleki, RSC Adv. 5, 75555 (2015)
M.A. Zolfigol, M. Safaiee, F. Afsharnadery, N. Bahrami-Nejad, S. Baghery, S. Salehzadeh, F. Maleki, RSC Adv. 5, 100546 (2015)
M.A. Zolfigol, M. Kiafar, M. Yarie, A. Taherpour, M. Saeidi-Rad, RSC Adv. 6, 50100 (2016)
M.A. Zolfigol, A. Khazaei, S. Alaie, S. Baghery, F. Maleki, Y. Bayat, A. Asghari, RSC Adv. 6, 58667 (2016)
A.R. Moosavi-Zare, M.A. Zolfigol, V. Khakyzadeh, C. Böttcher, M.H. Beyzavi, A. Zare, A. Hasaninejad, R. Luque, J. Mater. Chem. A 2, 770 (2014)
M.A. Zolfigol, S. Baghery, A.R. Moosavi-Zare, S.M. Vahdat, RSC Adv. 5, 32933 (2015)
M.A. Zolfigol, S. Baghery, A.R. Moosavi-Zare, S.M. Vahdat, H. Alinezhad, M. Norouzi, RSC Adv. 5, 45027 (2015)
M.A. Zolfigol, S. Baghery, A.R. Moosavi-Zare, S.M. Vahdat, H. Alinezhad, M. Norouzi, RSC. Adv. 4, 57662 (2014)
M. Ghorbani, S. Noura, M. Oftadeh, E. Gholami, M.A. Zolfigol, RSC Adv. 5, 55303 (2015)
M.A. Zolfigol, S. Baghery, A.R. Moosavi-Zare, S.M. Vahdat, J. Mol. Catal. A: Chem. 409, 216 (2015)
M.A. Zolfigol, H. Gholami, V. Khakyzadeh, Principles of Organic Synthesis with a New Approach, 3rd edn. (Bu-Ali Sina University Publishers, Hamedan, 2014), p. 26
J.M. Erhardt, J.D. Wuest, J. Am. Chem. Soc. 102, 6363 (1980)
T.J. Atkins, J. Am. Chem. Soc. 102, 6364 (1980)
J.M. Erhardt, E.R. Grover, J.D. Wuest, J. Am. Chem. Soc. 102, 6365 (1980)
All of the calculations were performed by: Spatran’ 10-Quantum Mechanics Program: (PC/x86) 1.1.0v4. 2011, Wave function Inc., USA
Acknowledgements
We thank Bu-Ali Sina University, Iran National Science Foundation (INSF, Grant No: 95831207) and National Elites Foundation for financial support to our research group.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zolfigol, M.A., Safaiee, M., Ebrahimghasri, B. et al. Application of novel nanostructured dinitropyrazine molten salt catalyst for the synthesis of sulfanylpyridines via anomeric based oxidation. J IRAN CHEM SOC 14, 1839–1852 (2017). https://doi.org/10.1007/s13738-017-1123-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-017-1123-z