Abstract
As promising structural materials, various tough hydrogels have been developed recently by incorporating various kinds of bonds. An important challenge is to use dual physical cross-linking to develop both toughness and self-recovery in a single material. Here we report smart, strain-responsive hydrogels composed of a fully physically linked agarose/poly(acrylic acid)-ferric ion (agar/PAAc-Fe3+) double network (DN) with high toughness and pH-sensitivity. These hydrogels were fabricated in a one-pot reaction to generate dual physical cross-linking through, first, a hydrogen-bonded cross-linked agarose network, and, second, a physically linked PAAc-Fe3+ network via Fe3+ coordination interactions. The DN hydrogels possessed high toughness, with breaking strain of 1130%, fast self-recovery properties in ambient conditions (100% recovery in 30 min) and self-healing properties (the healed hydrogels can be manually stretched up to 700% of their original length after self-healing for 60 h from the cut-off state). In addition, the hydrogels exhibited pH-sensitivity due to the dissociation of ionic coordinate bonds between –COO− ions of the PAAc chains and Fe3+ ions. Double-layer hydrogel strips with two different concentrations of PAAc formed a “C”-shaped material when initially immersed in pH 7 solution and then soaked in a pH 3 solution. These characteristics make the hydrogels attractive candidates for tissue engineering, soft actuators and flexible electronics.
Similar content being viewed by others
Introduction
Hydrogels are soft polymer materials with three-dimensional structures and high water retention; because of their hydrophilicity, biocompatibility and high toughness, they are the ideal substitute for biological tissue materials [1]. Wichterle and Lim synthesized the first sample of a hydrogel used as a biomedical material which was synthesized using poly-2-hydroxyethyl methacrylate that can be applied in the production of contact lenses [2]. Based on the biocompatibility and controllable physical, chemical and biological properties of the hydrogels, they have great application prospects as replacements for biological tissue materials, such as organ tissue replacement and wound dressings. Other examples include tissue engineering scaffolds [3], drug delivery [4, 5], biomolecule filters [6] and microfluidic valves [7].
With the development of research, various hydrogels that respond to external stimuli, such as pH, ionic strength, temperature and light, have attracted attention in applied biological tissue engineering. Human skin, muscle and other tissues are responsive to changes of the external environment. Therefore, the substitute materials for these tissues must also be environmentally sensitive.
Hydrogels that are responsive to pH can be classified into acidic or basic polymers. Both types exchange protons with their environments in a pH-dependent manner, and their behavior can, therefore, be controlled by the diffusion of hydrogen ions [8]. The pH-sensitive hydrogels are widely used in drug delivery research. When tissue develops an inflammation, infection or cancer, the pathological tissue experiences a pH environment that is different from that of the healthy one, allowing the medicine to be directionally delivered to the lesions without damage to the unaffected tissues.
Polymer materials with functional groups such as –COOH, –SO3H and –NH3 can become protonated and ionized. Many methods have been used to prepare pH-sensitive hydrogels. As noted above, pH-sensitive hydrogels can be classified into acidic (cationic) and basic (anionic) hydrogels according to the pH-sensitive functional groups. Poly(aspartic acid) [9, 10], poly(ethyl acrylic acid), poly(methacrylic acid) (PMAA) [11, 12], poly-sulfonamides [13] and 3-methylglutarylated poly(glycidol) [14] have been used to prepare anionic pH-sensitive polymeric materials. Due to containing amine functional groups, poly(β-amino ester) [15], poly(N,N-dimethylamino)ethyl methacrylate [16] and poly(l-histidine) [17] are deprotonated at basic pH and protonated or ionized at acidic pH. Amphoteric polymers with both cationic and anionic functional groups are ionized in alkaline conditions and protonated in acidic environments.
A highly flexible, tough and self-healable hydrogel based on poly(acrylic acid)/Fe3+ has been reported [18]. The dynamic ionic interaction endows the hydrogel with self-healing properties. Based on a di-block polymer backbone, where the first block is a copolymer of pH-sensitive ethyl acrylic acid monomers and hydrophobic butyl methacrylate or hexyl methacrylate monomers, a new pH-sensitive comb-like hydrogel was synthesized by Lin et al. [11]. It can enhance the intracellular delivery of therapeutic nucleic acids.
The pH-sensitive comb-like polymers degraded into small fragments upon incubation in an acidic solution (pH 5.8) due to hydrolysis of the hydrazone linkages connecting the hydrophobic/cationic grafts to the polymer backbone. This pH-sensitive function enabled the hydrogel to serve as a vehicle for effective intracellular delivery of therapeutic nucleic acids. Bonina et al. prepared a pH-sensitive hydrogel using chitosan and polyacrylamide [19]. In their study, the swelling rate of the hydrogel depended on the degree of cross-linking, and pH and ionic strength of the medium. Strehin et al. prepared a pH-sensitive hydrogel of chondroitin sulfate polyethylene glycol, which could be used in repairing cartilage tissue [20]. Furthermore, the strength, swelling properties and gelation kinetics of hydrogels can be controlled by changing the pH of the precursor solution [20]. Halacheva et al. prepared pH-sensitive hydrogels by blending cross-linked particles with high porosity, elasticity and flexibility in PMAA [21]. The mechanical properties of this hydrogel were greatly improved, indicating that pH-sensitive hydrogels are potential materials for tissue engineering. However, the preparation of these pH-sensitive hydrogels was complicated and time consuming.
As soft materials with high water content, most of hydrogels have weak mechanical properties [22]. Li et al. synthesized a hybrid dual cross-linked polyacrylic acid (PAAc) hydrogel by chemical cross-linking among PAAc chains and physical cross-linking through Fe3+ coordination interactions [23]. Chen et al. synthesized agar/PAMAAc-Fe3+ DN gels, consisting of agarose (agar) as the first physical network and PAMAAc-Fe3+ as the second chemical–physical network, which achieved high mechanical properties, good fatigue resistance and fast self-recovery [24]. Zhou et al. fabricated a high-performance hydrogel cross-linked by fully flexible cross-linking points of triblock copolymer micelles (Pluronic F127 diacrylate) and ionic interactions between Fe3+ and carboxyl groups [25]. Liu et al. designed hybrid dual cross-linked hydrogels with high toughness that can withstand in vivo gastric forces and achieve residence in the stomach for 7 days, allowing them to be applied in prolonged drug delivery [26]. Wang et al. prepared triple-network hydrogels composed of PAAc, agar and polyvinyl alcohol with good mechanical and self-healing properties [27].
However, there are few reports of hydrogels without chemical cross-linking that possess both high toughness and pH-sensitivity. Mechanical toughness and self-healing properties are two highly important characteristics in hydrogels for a wide variety of applications. While many hydrogels exhibit one of these properties, very few hydrogels possess both, and it remains an ongoing challenge to develop hydrogels that incorporate both mechanical toughness and self-healing properties within a single material. In our previous work, we have successfully fabricated dual physically cross-linked agar/PAAc-Fe3+ double network (DN) hydrogels, with strong interfacial interactions between agar chains and dynamic coordination bonds facilitated by Fe3+ ions, which have strong coordination ability relative to other metal ions [28].
Due to this dual physical cross-linking, the fabricated agar/PAAc-Fe3+ DN hydrogels exhibited very favorable mechanical and self-healing properties. In this study, we report a “one-pot” preparation strategy for fabricating smart hydrogels with inhomogeneous structures and pH-sensitive properties. The relationship between mechanical and swelling properties and the pH environment is discussed systematically. The agar/PAAc-Fe3+ DN gels exhibit excellent multi-stimuli-responsive properties and controllable shape deformation behaviors, with potential uses in the fields of soft robotics and actuators.
Experimental
Materials
The following materials were used: agarose (agar, Invitrogen, USA, biochemical reagent grade); acrylic acid (AAc, Sinopharm Chemical Reagent Co., Ltd., Shanghai, China, chemically pure, content ≥ 98.0%); Fe(NO3)3·9H2O (ferric nitrate, Sinopharm Chemical Reagent Co., Ltd., Shanghai, China, analytically pure); 2-ketoglutaric acid (KA, Sinopharm Chemical Reagent Co., Ltd., Shanghai, China, biochemical reagent grade); hydrochloric acid (HCl, Sinopharm Chemical Reagent Co., Ltd., Shanghai, China, analytically pure); and sodium hydroxide (NaOH, Sinopharm Chemical Reagent Co., Ltd., Shanghai, China, analytically pure).
Preparation method
agar/PAAc-Fe3+ DN hydrogels were synthesized in a one-pot reaction. Agar was added to a 250-mL, three-neck, round-bottomed flask to form a homogeneous solution at 65 °C. AAc, ferric nitrate (the ionic cross-linker) and KA (the ultraviolet (UV)–light initiator) were added to the solution to form a mixed aqueous solution, which was then poured into a homemade, rectangular reaction-cell consisting of two glass plates with smooth surfaces separated with a hollow silicone-rubber spacer of ~ 2.6 mm thickness. The sealed reaction-cell was allowed to stand at room temperature for 1 h. Then, AAc was polymerized and physically cross-linked upon UV–light irradiation at room temperature for 5 h in the presence of KA as the UV initiator and ferric ions as the ionic cross-linker. As the control group, PAAc-Fe3+ single-network (SN) hydrogels were prepared by the same process except without the addition of agar. AAc monomers, KA and ferric nitrate were added into a reactor to form a mixed aqueous solution, which was then exposed to UV irradiation for 5 h to fabricate PAAc-Fe3+ SN hydrogels. Agar SN hydrogels were prepared by a similar process, i.e. agar and water were added to a reactor to form a homogeneous solution at 65 °C, followed by cooling the solution at room temperature for 1 h to form agar SN.
For purposes of comparison with our newly developed one-pot method, we also used the two-step method to prepare agar/PAAc-Fe3+ DN hydrogels. The two-step method was different from the one-pot method for agar/PAAc-Fe3+ DN hydrogels. The two-step method still follows sequential polymerization steps to form two networks independently. First, the agar network was formed by a heating–cooling process, during which the components for the second network (AAc and UV initiator) were filled into the first (agar) network. Then, exposed to UV irradiation for 5 h, the PAAc network was formed. Finally, agar/PAAc-Fe3+ DN hydrogels were obtained by soaking the agar/PAAc DN hydrogels in a solution of Fe3+ ions for 1 day.
To prepare double-layered agar/PAAc-Fe3+ hydrogel strips, an agar/PAAc-Fe3+ hydrogel sample with a higher concentration of PAAc (4.59 mol L−1) was dyed blue and then attached to another agar/PAAc-Fe3+ hydrogel sample with a lower concentration of PAAc (3 mol L−1). The Fe3+ concentration of both samples was 0.015 mol L−1.
Characterization
Tensile
Uniaxial tensile tests of the as-prepared hydrogels (40 mm in length, 10 mm in width and 2.6 mm in thickness) were carried out using a universal tensile tester (CMT4000, MTS Co. Ltd., China) equipped with a 1 kN load cell at a crosshead speed of 50 mm/min. The tensile stress was calculated as follows:
where l and l0 are the sample lengths during stretching and before stretching, respectively. The tensile stress was calculated as follows:
where F is the loading force and A0 is the original specimen cross-sectional area.
Compression
Uniaxial compression tests of the cylindrical as-prepared hydrogels (13 mm in diameter and 2.6 mm in thickness) were carried out using a universal tensile tester (CMT4000, MTS Co. Ltd., China) equipped with a 1 kN load cell at a crosshead speed of 0.26 mm/min. The stress–strain curve (strain range of 5–10%) was linear fitted to obtain a straight line, the slope of which was the value of the compression modulus.
Self-healing
The cylindrical agar/PAAc-Fe3+ DN hydrogel samples were cut into two using a knife. Then, the two freshly cut surfaces were placed together (without adding any chemicals) within plastic syringes (with similar dimensions to the as-prepared hydrogel samples). To avoid water evaporation, the plastic syringes were wrapped in polyethylene films and stored in sealed polyethylene bags at stipulated temperatures over specific times. After the allotted time periods, the healed agar/PAAc-Fe3+ hydrogels were subjected to mechanical measurements at ambient temperature to study their self-healing properties.
Swelling ratio
A hydrogel sample was soaked in a solution for a certain period of time until the weight of the sample kept constant at room temperature (25 °C). Then, the mass of the soaked sample was measured, and the hydrogel sample at swelling equilibrium was dried in an electric drying oven. The swelling ratio is defined as the weight of the equilibrium gel divided by the weight of the as-prepared hydrogel. The swelling ratio S of the hydrogel sample was calculated by the following formula:
where W is the weight of the soaked sample, and W0 is the weight of the dried sample.
pH response
The agar/PAAc-Fe3+ DN hydrogels were studied gravimetrically as a function of pH in the range from 1 to 13. The samples were soaked in a large amount of deionized water at each predetermined pH for 30 min. The pH was controlled by NaOH or HCl solutions. The samples were taken out and the surface water was removed using filter paper to measure the sample mass. Then, the samples were tested.
Results and discussion
Synthesis of agar/PAAc-Fe3+ DN hydrogels and optimized conditions
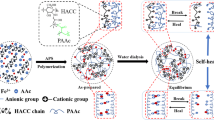
Scheme 1a schematically illustrates the simple one-pot fabrication procedure, with all of the reactants (agar, AAc monomers, UV initiator KA and ionic cross-linker ferric nitrate) added to a single reactor, followed by a three-step synthetic process of heating, photo-polymerization and cooling. Scheme 1b illustrates the double helix structure of agar. Scheme 1c shows the cross-linking in PAAc macromolecular chains by ionic bonding of ferric ions. The simple one-pot approach ensures a short preparation time and allows for easy control of the constituent concentrations to optimize the properties of the DN hydrogels. In the agar/PAAc-Fe3+ hydrogels, the rigid and brittle polymer agar acts as the first network, and the soft and ductile polymer PAAc as the second network.
To optimize the mechanical properties of the agar/PAAc-Fe3+ DN hydrogels, the influence of the molar ratio of Fe3+ and AAc was studied. The agar content (macromolecular repeat unit) was 0.05 mol L−1.
As shown in Fig. 1a, the effect of varying the ferric ion concentration highlights the key role that they play in the outstanding mechanical properties of the agar/PAAc-Fe3+ hydrogels. The tensile strength at break was improved with the gradual increase of Fe3+ content from 0.003 to 0.015 mol L−1. Subsequently, a decline of the mechanical properties was observed for the hydrogels with Fe3+ content higher than 0.015 mol L−1.
At moderate levels, increasing the content of ferric ions facilitated the polymerization of PAAc and increased the cross-linking density of the hydrogels, which enabled more stress to be sustained by the sample. However, with a further increase of the ferric ion content, it retarded the radical polymerization and reduced the molecular weight of PAAc, thus degrading the mechanical properties of the hydrogels [29, 30].
As shown in Fig. 1b, the mechanical properties of the hydrogels were also found to be dependent on the AAc concentration. The variation in the AAc content was found to significantly influence the elastic modulus and tensile strength of the agar/PAAc-Fe3+ hydrogels. The tensile strength, elastic modulus and elongation were improved with the gradual increase of AAc concentration from 1.02 to 4.59 mol L−1.
The agar/PAAc-Fe3+ hydrogels with an AAc concentration of 4.59 mol L−1 were found to have good deformation characteristics and tolerate an elongation to more than 11 times of their initial length, while those with an AAc concentration of 5.10 mol L−1 could tolerate less than an eightfold elongation.
When the AAc concentration was 4.59 mol L−1, the stress–strain curve of the agar/PAAc-Fe3+ hydrogels showed that yielding occurred when the strain was 100%. This high ability to withstand strain can be ascribed to the interactions between the two networks. The strength of DN hydrogels increased when the molar ratio of the second network to the first one increased. However, higher concentrations of the second network also make the hydrogel more susceptible to macroscopic breakage under the loading process.
As shown in Fig. 1b, compared with agar SN hydrogels (8 kPa) and PAAc-Fe3+ SN hydrogels (30 kPa), the agar/PAAc-Fe3+ DN hydrogels exhibited a much higher tensile strength of 320 kPa, even though the DN hydrogels contained the same total content of the respective components as the SN hydrogels. The failure stress and strain of the agar/PAAc-Fe3+ DN hydrogels were 320 kPa and 1130%, respectively. They were much higher than those of the agar SN hydrogels (4.93 kPa and 12%) and PAAc-Fe3+ SN hydrogels (34.76 kPa and 428%).
As shown in Fig. 1b, when the molar concentration of the second network [PAAc (4.59 mol L−1) linked through Fe3+ coordination interactions] was 90 times higher than the first network [agar (0.05 mol L−1)], the resulting structural parameters of the two networks endow the hydrogels with optimum mechanical properties. Additionally, the monomer and ferric ion concentrations during the polymerization also influenced the macromolecular weight. It was found that the agar/PAAc-Fe3+ hydrogels with a moderate PAAc content exhibited a balanced mechanical performance. Therefore, all of the agar/PAAc-Fe3+ DN hydrogels studied in the following experiments were chosen to have ferric ion and AAc concentrations of 0.015 mol L−1 and 4.59 mol L−1, respectively. In summary, the DN structure remarkably strengthens the mechanical properties of the agar/PAAc-Fe3+ hydrogel.
Figure 2a, b shows SEM micrographs of agar SN hydrogels at two different magnifications. Agar is a thermo-sensitive material; the sol–gel reaction of agar is reversible. When cooled to below the critical transformation temperature, agar molecular chains transform from linear into a spiral structure, and this structure enables agar to be molded. As shown in Fig. 2a, b, due to the high water content of up to 98%, the pore size of agar was relatively as large as 50 µm, indicating a low-density network structure. This lack of compactness led to weak mechanical properties of the agar SN hydrogels.
Figure 2c, d shows SEM micrographs of PAAc-Fe3+ SN hydrogels at two different magnifications. Compared with agar SN hydrogel, the pore size of the PAAc-Fe3+ network was smaller, which means that the PAAc-Fe3+ hydrogels have a more compact structure. However, the network of the PAAc hydrogel cross-linked by Fe3+ is less uniform and homogeneous, and stress can easily accumulate during the process of load-bearing.
Figure 2e, f shows SEM micrographs of agar/PAAc-Fe3+ hydrogels at low and high magnification. The SEM micrographs indicate that the double network of the hydrogels had been successfully synthesized. Due to the presence of the second PAAc-Fe3+ network, which increased the relative cross-link density of the hydrogel structure, the agar/PAAc-Fe3+ DN hydrogels appeared to have more compact porous structures with an average pore size of about 10 µm. Moreover, the DN hydrogels showed a homogeneous network structure that was more porous in character, in which the holes formed after the remove of the absorbed water. The rigid agar chains formed a skeleton while the soft and tough PAAc chains were able to dissipate energy to prevent fracture. The rigid agar molecular chains thus mitigated the inhomogeneity of the PAAc network, and the DN structure greatly improved the mechanical properties of the agar/PAAc-Fe3+ hydrogels.
Self-recovery of agar/PAAc-Fe3+ DN hydrogels
The dual physically cross-linked agar/PAAc-Fe3+ DN hydrogels showed excellent self-recovery properties under a range of recovery conditions. As shown in Table 1, damaged samples were placed in three recovery environments for different recovery times, and the mechanical properties were tested to calculate the recovery ratio. In ambient conditions, for resting times exceeding 30 min, the recovery of peak stress and dissipated energy reached 100%. Specifically, the agar/PAAc-Fe3+ hydrogels achieved rapid recovery to ≈ 24% stiffness (30 kPa) and ≈ 33% toughness (51 kJ m−3) after 15 min, and a maximal recovery of ≈ 97% stiffness (104 kPa) and ≈ 138% toughness (212 kJ m−3) after 60 min.
The as-prepared agar/PAAc-Fe3+ DN hydrogels were tan and transparent (Fig. 3). For self-healing tests, samples of the agar/PAAc-Fe3+ DN gels were dyed blue using methylene blue (Fig. 3a). As shown in Fig. 3c, two cut samples—one of the as-prepared hydrogel and one of the dyed hydrogel—were placed together without any external stimuli. After a healing time of 60 h, the shear mark blurred and the two samples were melded into one (Fig. 3d). The healed agar/PAAc-Fe3+ DN hydrogels could be manually stretched up to 700% of their original length without necking phenomena and cracks (Fig. 3e).
Images of self-healing properties of agar/PAAc-Fe3+ DN hydrogels: cut cuboid samples (a, b), cut cuboid samples placed together (c), free-standing healed samples after incubation without any external stimuli for 60 h (d), healed agar/PAAc-Fe3+ DN gels could be stretched over seven times (e), stretched over five times from a knotted state (f) stretched over three times from a twisted state (g), Variation of mechanical properties between initial and self-healed agar/PAAc-Fe3+ DN hydrogels (h), variations of tensile strength, elongation-at-break and Young’s modulus of initial and self-healed agar/PAAc-Fe3+ DN hydrogels (i) and as a control, Agar/PAAc-Fe3+ DN hydrogels fabricated by soaking Agar/PAAc DN hydrogels in a solution of Fe3+ ions for 1 day failed to self-heal (j) (concentrations of Fe3+ and AAc were 0.015 mol L−1 and 4.59 mol L−1, respectively)
In addition to the high stretchability, the agar/PAAc-Fe3+ DN hydrogels could also be flexibly knotted and twisted, providing malleability into diverse shapes, outstanding processability and extraordinary mechanical performance. As seen in Fig. 3f, the healed hydrogels could be knotted without forming cracks and could even be further stretched. After releasing the tensile force, the hydrogels recovered well and could be easily unknotted. A repeat process of manual twisting could then be conducted without breaking the hydrogel (Fig. 3g).
Furthermore, the self-healed hydrogels could be stretched to 774% of their initial length (Fig. 3h). As can be observed in Fig. 3i, the agar/PAAc-Fe3+ hydrogels after self-healing attained a failure stress of 227 kPa and a Young’s modulus of 43.9 kPa. The DN hydrogels thus exhibited good self-healing properties.
As a control, agar/PAAc-Fe3+ hydrogels were fabricated by soaking agar/PAAc hydrogels in a solution of Fe3+ ions. As shown in Fig. 3j, the self-healing properties were weaker than those of the DN hydrogels fabricated by our one-pot method. The Fe3+ ions produced more stable tridentate complexes with –COO− ions. Thus, in the hydrogels produced by soaking in Fe3+, the free Fe3+ ions were disfavored from forming new ionic complexes between interfaces; therefore, these hydrogels failed to self-heal.
The self-healing of the hydrogels at room temperature occurred via three processes: free ion diffusion, the dynamic migration of Fe3+ ions, and the formation of new ionic complexes between interfaces. Through dynamic ion migration, the Fe3+ ions formed complexes with the –COO− groups of PAAc chains located on different fracture surfaces, thus rebuilding the PAAc-Fe3+ network, and promoting self-healing. Additionally, with the healing of the PAAc-Fe3+ network, the PAAc chains formed entangled structures, which further facilitated the reestablishment of hydrogen bonds between agar chains.
In the pH range from 3 to 11, the agar/PAAc-Fe3+ hydrogels exhibited relatively stable mechanical properties with respect to tensile strength and elongation-at-break, as well as good healing properties. Quantitative research into the pH dependence of the healing performance will be conducted in the near future.
Mechanical properties of agar/PAAc-Fe3+ DN gels at different pH solutions
After being soaked for 30 min in solutions with different pH, the samples were tested to obtain their pH-dependent compressive properties, and the compression moduli are shown in Fig. 4a. As can be seen, the compression modulus of the gels increased with increasing pH from 1 to 3, and achieved a maximum value of 117 kPa at pH 3. In the pH range from 3 to 13, the compression modulus of the gels continuously decreased with increasing pH of the solution. Therefore, the compressive properties in acid solution were greater than in neutral and basic solutions.
To study the reason of the influence of pH, samples of agar/PAAc-Fe3+ hydrogels were tested for tensile properties to obtain the stress–strain diagram after being soaked for 30 min in different pH solutions (Fig. 4b). At pH 3, the stress–strain curve displays a significant yield phenomenon at a strain of about 50%. Figure 4c shows the variation of tensile strength and elongation-at-break values calculated from the tensile stress–strain curves of Fig. 4b in different pH solutions. As shown in Fig. 4c, the tensile strength and elongation-at-break values of the gels increased with increasing pH from 1 to 3 and decreased with the further increase of pH from 3 to 13. At pH 3, the samples displayed excellent mechanical properties: the agar/PAAc-Fe3+ hydrogel reached a tensile strength of 220 kPa and elongation-at-break of 500%.
The PAAc molecular chains of agar/PAAc-Fe3+ DN hydrogels contain a large amount of –COO− groups, a hydrolyzable group that can undergo several reversible reactions (ionization and complexation) in solution. These reversible reactions result in an ion concentration gradient between the interior and exterior of the hydrogel samples, and this gradient endows the hydrogels with pH-sensitivity.
As has been described, the mechanical properties of the hydrogels were weakened after soaking in alkaline media. Likewise, when the pH of the solution was as low as 1, corresponding to strongly acidic conditions, the mechanical properties of the hydrogels were also weakened. Therefore, soaked in a mildly acidic medium (appropriate acidity) was benefit for the mechanical properties, while highly acidic and alkaline conditions weaken the mechanical properties of agar/PAAc-Fe3+ DN hydrogels.
In the alkaline solutions, the –COOH groups of PAAc molecular chains were ionized by reaction with the OH− in the solution to form carboxylate ions –COO−. Due to mutual electrostatic repulsion between –COO− ions, the bonding force between molecular chains of agar/PAAc-Fe3+ DN hydrogels was weakened. The higher the pH value of the solution, the higher is the concentration of OH− in the solution. Then, in turn, the greater the amount of ionized –COO− in the hydrogel network, the greater the electrostatic repulsion between chains in the network, which weakens the mechanical properties of agar/PAAc-Fe3+ DN hydrogels.
Moreover, in alkaline condition, a large amount of OH− prevents the coordination between Fe3+ ions and –COO− functional groups. The Fe3+ ions tend to combine with OH− ions to form Fe(OH)3, which weakened the mechanical properties. The decrease in the tensile strength and elongation-at-break values of the gels was especially significant with the increase of pH from 11 to 13.
In acidic condition, a large amount of H+ ions exists in solution, which prevents Fe3+ ions to combine with OH− moieties. The ionic coordinate bonds between the –COO− functional groups of PAAc molecular chains and the Fe3+ ions increase the bonding force of the hydrogel network and reinforce the hydrogel’s mechanical properties. The mechanical properties of the hydrogel samples are optimized at pH 3. However, at pH 1, the over-abundance of H+ ions prevents ionization of –COOH groups, and reduced the extent of cross-linking by ionic bonding of ferric ions with these functional groups.
As shown in Fig. 5, when samples of agar/PAAc-Fe3+ DN hydrogels were immersed in solution with different pH values, the swelling ratio of the samples increased with increasing the soaking time. As can be seen, the swelling ratio of the gel gradually increased as the pH value of the solutions increased from 1 to 13 after the same duration of soaking time. The swelling ratio of the hydrogel increased the fastest, and reached the highest value (17), at pH 13. Compared with the neutral environment (pH = 7), the swelling ratio of the hydrogel was suppressed in the acidic solutions (pH = 1 and 3) but enhanced in the alkaline conditions (pH = 11 and 13).
An ionic interaction between the Fe3+ ions and –COO− functional groups in the PAAc molecular chains led to formation of ionic coordination bonds between them. Every three carboxylate groups –COO− coordinated with one Fe3+ ion to form ionically bonded cross-links, which shrank the molecular chains due to the enhanced bonding force of the hydrogel network. With the increasing of bonding force, the swelling ratio declined.
In the alkaline media (pH = 11 and 13), the –COOH groups in the PAAc molecular chains were ionized by reacting with OH− ions to form carboxylate ions –COO− functional groups. Electrostatic repulsion among the –COO− groups weakened the bonding force of the molecular networks, causing the swelling ratio to increase. As can be seen from Fig. 6, the stronger the alkalinity of the solution, the greater the promotion of the swelling ratio.
a Images of samples of agar/PAAc-Fe3+ DN hydrogels swelled at solutions with different pH values and b schematic presentation of chemical structure and bonding of agar/PAAc-Fe3+ DN hydrogels formed at different pH conditions (concentrations of Fe3+ and AAc were 0.015 mol L−1 and 4.59 mol L−1, respectively)
Figure 6a shows the swelling ratios of the samples soaked in solutions with two different pH values. After soaking for 30 min, the swelling ratio of the sample at pH 3 was much lower than that of the sample at pH 13. Figure 6b shows a schematic of the chemical mechanism of the swelling of agar/PAAc-Fe3+ DN hydrogels at different pH conditions.
As stated above, in the pH range from 1 to 13, the swelling ratio of the agar/PAAc-Fe3+ DN hydrogels continuously increased with increasing pH. At lower pH, the –COO− groups coordinated with Fe3+ ions to form tridentate complexes. At pH 3, the ionic bonding coordination of Fe3+ within the hydrogel networks was maximized, which restricted the swelling of the hydrogels. With the increase of pH, the Fe3+ ions produced a mixture of mono-, bi-, or tridentate complexes with –COO− functional groups among which the tridentate complexes were the most favorable one for enhancing the mechanical properties of the hydrogels [31, 32].
In the alkaline condition, a large amount of OH− ions was combined with Fe3+ ions, which led to the dissociation of ionic coordinate bonds. At pH 13, the reaction of OH− with –COOH groups produced carboxylate (–COO−) functional groups. Electrostatic repulsion among these –COO− functional groups weakened the bonding force of the molecular networks. The intramolecular electrostatic repulsion, therefore, favors the swelling of the agar/PAAc-Fe3+ DN hydrogels.
The concentration of PAAc influences the level of ionic coordination bonding or electrostatic repulsion. In the sample with higher concentration of PAAc, strong ionic coordination bonds formed in solution at pH = 3, while strong electrostatic repulsion occurred at pH = 13. This characteristic makes it possible to design a double-layered hydrogel material with an intelligent pH response, as shown in Fig. 7a, b.
The agar/PAAc-Fe3+ DN hydrogels with different concentrations of PAAc showed different swelling ratios in a solution with pH 3. In the hydrogel with a high concentration of PAAc, a large number of the –COO− functional groups were formed in the acidic condition and coordinated with Fe3+ ions. Therefore, the hydrogel with a high concentration of PAAc has stronger ionic coordination bonding than the hydrogel with a low PAAc concentration. The difference in swelling ratio between the two layers caused the material to form the shape of the letter “C”.
Conclusion
A dual physically cross-linked agar/PAAc-Fe3+ DN hydrogel, prepared by a “one-pot” method, has a self-healing ability, which allows it to heal a fractured surface by reconstructing the reversible physical cross-links without the need for a temperature change, chemical additives or other external stimuli. After healing, the hydrogel retains excellent mechanical properties. The dual physically cross-linked agar/PAAc-Fe3+ DN hydrogel also has pH-responsive properties in the range of pH = 3–13. The swelling ratio increased with increasing pH value while the mechanical properties deteriorated. In a solution with pH = 3, hydrogels with different concentrations of AAc have different swelling ratios. This characteristic allows agar/PAAc-Fe3+ DN hydrogels with different AAc concentrations to be assembled into a material with intelligent pH response. The pH-sensitive properties are expected to enable the hydrogel to be applied in tissue engineering.
References
Gong JP, Katsuyama Y, Kurokawa T, Osada Y (2003) Double-network hydrogels with extremely high mechanical strength. Adv Mater 15:1155–1158
Wichterle O, Lim D (1960) Hydrophilic gels for biological use. Nature 185:117–118
Salgado CL, Oliveira MB, Mano JF (2012) Combinatorial cell-3D biomaterials cytocompatibility screening for tissue engineering using bioinspired superhydrophobic substrates. Integr Biol 4:318–327
Duan J, Liang X, Cao Y, Wang S, Zhang L (2015) High strength chitosan hydrogels with biocompatibility via new avenue based on constructing nanofibrous architecture. Macromolecules 48:2706–2714
Li L, Yan B, Yang J, Chen L, Zeng H (2015) Novel mussel-inspired injectable self-healing hydrogel with anti-biofouling property. Adv Mater 27:1294–1299
Thomas PC, Cipriano BH, Raghavan SR (2011) Nanoparticle-crosslinked hydrogels as a class of efficient materials for separation and ion exchange. Soft Matter 7:8192–8197
Chiu H-C, Lin Y-W, Huang Y-F, Chuang C-K, Chern C-S (2008) Polymer vesicles containing small vesicles within interior aqueous compartments and ph-responsive transmembrane channels. Angew Chem Int Edit 47:1875–1878
Lim HL, Hwang Y, Kar M, Varghese S (2014) Smart hydrogels as functional biomimetic systems. Biomater Sci 2:603–618
Kim JH, Oh YT, Lee KS, Yun JM, Park BT, Oh KT (2011) Development of a pH-sensitive polymer using poly(aspartic acid-graft-imidazole)-block-poly (ethylene glycol) for acidic pH targeting systems. Macromol Res 19:453–460
Liu H, Liu M, Ma L, Chen J (2009) Thermo-and pH-sensitive comb-type grafted poly (N,N-diethylacrylamide-co-acrylic acid) hydrogels with rapid response behaviors. Eur Polym J 45:2060–2067
Lin YL, Jiang G, Birrell LK, EI-Sayed MEH (2010) Degradable, pH-sensitive, membrane-destabilizing, comb-like polymers for intracellular delivery of nucleic acids. Biomaterials 31:7150–7166
Xu Q, Huang W, Jiang L, Lei Z, Li X, Deng H (2013) KGM and PMAA based pH-sensitive interpenetrating polymer network hydrogel for controlled drug release. Carbohyd Polym 97:565–570
Ravazzolo E, Salmaso S, Mastrotto F, Bersani S, Gallon E, Caliceti P (2013) pH-responsive lipid core micelles for tumour targeting. Eur J Pharm Biopharm 83:346–357
Yuba E, Harada A, Sakanishi Y, Watarai S, Kono K (2013) A liposome-based antigen delivery system using pH-sensitive fusogenic polymers for cancer immunotherapy. Biomaterials 34:3042–3052
Kim MS, Hwang SJ, Han JK, Choi EK, Park HJ, Kim JS, Lee D-S (2006) pH-responsive PEG-poly(β-amino ester) block copolymer micelles with a sharp transition. Macromol Rapid Comm 27:447–451
Hu Y, Wang J, Zhang H, Jiang G, Kan C (2014) Synthesis and characterization of monodispersed P(St-co-DMAEMA) nanoparticles as pH-sensitive drug delivery system. Mater Sci Eng C Mater Biol Appl 45:1–7
Li Z, Qiu L, Chen Q, Hao T, Qiao M, Zhao H, Zhang J, Hu H, Zhao X, Chen D, Mei L (2015) pH-sensitive nanoparticles of poly (l-histidine)-poly (lactide-co-glycolide)-tocopheryl polyethylene glycol succinate for anti-tumor drug delivery. Acta Biomater 11:137–150
Zhou H, Zhang M, Cao J, Yan B, Yang W, Jin X, Ma A, Chen W, Ding X, Zhang G, Luo C (2017) Highly flexible, tough, and self-healable hydrogels enabled by dual cross-linking of triblock copolymer micelles and ionic interactions. Macromol Mater Eng 302:1600352
Bonina P, Petrova T, Manolova N (2004) pH-sensitive hydrogels composed of chitosan and polyacrylamide-preparation and properties. J Bioact Compat Polym 19:101–116
Strehin I, Nahas Z, Arora K, Nguyen T, Elisseeff J (2010) A versatile pH sensitive chondroitin sulfate-PEG tissue adhesive and hydrogel. Biomaterials 31:2788–2797
Halacheva SS, Freemont TJ, Saunders BR (2013) pH-responsive physical gels from poly(meth) acrylic acid-containing crosslinked particles: the relationship between structure and mechanical properties. J Mater Chem B 1:4065–4078
Yang CH, Wang MX, Haider H, Yang JH, Sun J-Y, Chen YM, Zhou J, Suo Z (2013) Strengthening alginate/polyacrylamide hydrogels using various multivalent cations. ACS Appl Mater Interfaces 5:10418–10422
Li X, Zhao Y, Li D, Zhang G, Long S, Wang H (2017) Hybrid dual crosslinked polyacrylic acid hydrogels with ultrahigh mechanical strength, toughness and self-healing properties via soaking salt solution. Polymer 121:55–63
Chen Q, Yan X, Zhu L, Chen H, Jiang B, Wei D, Huang L, Yang J, Liu B, Zheng J (2016) Improvement of mechanical strength and fatigue resistance of double network hydrogels by ionic coordination interactions. Chem Mater 28:5710–5720
Zhou H, Jin X, Yan B, Li X, Yang W, Ma J, Zhang X, Li P, Ding X, Chen W (2017) Mechanically robust, tough, and self-recoverable hydrogels with molecularly engineered fully flexible crosslinking structure. Macromol Mater Eng 302:1700085
Liu J, Pang Y, Zhang S, Cleveland C, Yin X, Booth L, Lin J, Lee YL, Mazdiyasni H, Saxton S, Kirtane AR, Von Erlach T, Rogner J, Langer R, Traverso G (2017) Triggerable tough hydrogels for gastric resident dosage forms. Nat Commun 8:124
Wang Y, Niu J, Hou J, Wang Z, Wu J, Meng G, Liu Z, Guo X (2018) A novel design strategy for triple-network structure hydrogels with high-strength, tough and self-healing properties. Polymer 135:16–24
Li X, Yang Q, Zhao Y, Long S, Zheng J (2017) Dual physically crosslinked double network hydrogels with high toughness and self-healing properties. Soft Matter 13:911–920
Wei Z, He J, Liang T, Oh H, Athas J, Tong Z, Wang C, Nie Z (2013) Autonomous self-healing of poly(acrylic acid) hydrogels induced by the migration of ferric ions. Polym Chem 4:4601–4605
Hernández S, Papp JK, Bhattacharyya D (2014) Iron-based redox polymerization of acrylic acid for direct synthesis of hydrogel/membranes, and metal nanoparticles for water treatment. Ind Eng Chem Res 53:1130–1142
Hu Y, Du Z, Deng X, Wang T, Yang Z, Zhou W, Wang C (2016) Dual physically cross-linked hydrogels with high stretchability, toughness, and good self-recoverability. Macromolecules 49:5660–5668
Zheng SY, Ding H, Qian J, Yin J, Wu ZL, Song Y, Zheng Q (2016) Metal-coordination complexes mediated physical hydrogels with high toughness, stick–slip tearing behavior, and good processability. Macromolecules 49:9637–9646
Acknowledgements
The authors acknowledged with gratitude the support from the National Natural Science Foundation of China (NSFC) (Contract no. 51603065) and from the Open Fund of Hubei Provincial Key Laboratory of Green Materials for Light Industry (Contract no. 201806A02).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Li, X., Zhang, Y., Yang, Q. et al. Agar/PAAc-Fe3+ hydrogels with pH-sensitivity and high toughness using dual physical cross-linking. Iran Polym J 27, 829–840 (2018). https://doi.org/10.1007/s13726-018-0657-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-018-0657-y