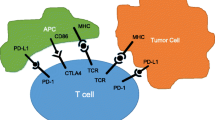
Abstract
Purpose of Review
The purpose of this review is to update readers on recent advancements in the use of immune checkpoint inhibitors for the treatment of ovarian, uterine, and cervical cancers.
Recent Findings
Immunotherapy has emerged as a novel therapeutic paradigm in the treatment of gynecologic malignancies. Currently, immune checkpoint inhibitors are approved for use across five solid malignancies, with recent approval of pembrolizumab in patients with MMR-deficient, recurrent, solid tumors in a disease site agnostic fashion. Phase 3 clinical trials are being conducted in the gynecologic cancer arena to determine if checkpoint inhibition will improve oncologic outcomes. Positive signals have been identified in ovarian cancer cohorts, both as single agents and in combination with other agents. It is anticipated that immunotherapy will be effective in MMR-deficient endometrial cancers, and trials are in development to explore these agents in the front line. Furthermore, the HPV-driven biology of cervical cancer suggests that immune checkpoint inhibition may lead to clinical benefit.
Summary
Immune checkpoint inhibitors represent a dynamic and exciting opportunity for patients with limited therapeutic options. We eagerly await the results of ongoing phase 3 clinical trials that will inform practice patterns. In addition, emphasizing translational end-points informing patient selection and response is critical.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Sumire V, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms †. Mol Cell Biol. 2005;25(21):9543–53. https://doi.org/10.1128/MCB.25.21.9543-9553.2005.
Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65(3):1089–96.
Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science [Internet]. 1996;271(5256):1734–6. (80) Available from:. https://doi.org/10.1126/science.271.5256.1734.
Siegel RL, Miller KD, Jemal A. Cancer Statistics , 2017;67(1):7–30.
Pujade-Lauraine E, Hilpert F, Weber B, Reuss A, Poveda A, Kristensen G, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized phase III trial. J Clin Oncol. 2014;32(13):1302–8. https://doi.org/10.1200/JCO.2013.51.4489.
Eskander RN, Tewari KS. Immunotherapy: an evolving paradigm in the treatment of advanced cervical cancer. Clin Ther [Internet]. 2015;37(1):20–38. Available from:. https://doi.org/10.1016/j.clinthera.2014.11.010.
• Ding M, Mei-jiao G. Immune effect of tumor-infiltrating lymphocytes and its relation to the survival rate of patients with ovarian malignancies. J Tongji Med Univ [internet]. 1991;11(4):235–9. https://doi.org/10.1007/BF02888158. One of the earliest studies suggesting the prognostic importance of tumor-infiltrating lymphocytes and outcomes in ovarian cancer
Li J, Wang J, Chen R, Bai Y, Lu X. The prognostic value of tumor-infiltrating T lymphocytes in ovarian cancer. Oncotarget. 2017;8(9):15621–31. https://doi.org/10.18632/oncotarget.14919.
Makrigiannakis A, Gray H. Intratumoral T cells, recurrence, and survival in epithelial ovarian. Cancer. 2003:203–13.
Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol [Internet]. 2011;8(3):151–60. Available from:. https://doi.org/10.1038/nrclinonc.2010.223.
Vergote I, Tropé CG, Amant F, Kristensen GB, Ehlen T, Johnson N, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med [Internet]. 2010;363(10):943–53. Available from:. https://doi.org/10.1056/NEJMoa0908806.
Melamed A, Hinchcliff EM, Clemmer JT, Bregar AJ, Uppal S, Bostock I, et al. Trends in the use of neoadjuvant chemotherapy for advanced ovarian cancer in the United States. Gynecol Oncol [Internet]. 2016;143(2):236–40. Available from:. https://doi.org/10.1016/j.ygyno.2016.09.002.
Tap WD, Wainberg ZA, Anthony SP, Ibrahim PN, Zhang C, Healey JH, et al. Structure-guided blockade of CSF1R kinase in tenosynovial giant-cell tumor. N Engl J Med [Internet]. 2015;373(5):428–37. Available from:. https://doi.org/10.1056/NEJMoa1411366.
Jochems C, Fantini M, Fernando RI, Kwilas AR, Donahue RN, Lepone LM, et al. The IDO1 selective inhibitor epacadostat enhances dendritic cell immunogenicity and lytic ability of tumor antigen-specific T cells. Oncotarget [Internet]. 2014;7(25):37762–72. Available from: http://www.oncotarget.com/abstract/9326
•• Lee J, Cimino-mathews A, Peer CJ, Zimmer A, Lipkowitz S, Annunziata CM, et al. Safety and clinical activity of the programmed death-ligand 1 inhibitor durvalumab in combination with poly (ADP-ribose) polymerase inhibitor olaparib or vascular endothelial growth factor receptor 1–3 inhibitor cediranib in women’s cancers: a dose-escalation, phase I study. 2017; One of the first trials describing safety and preliminary efficacy of combination checkpoint inhibition and PARP inhibition.
Sundarapandiyan, K, Zhao, B, Vitale, L, O’Neill, T, Ramakrishna, V., Marsh, H, Keler T. Characterization of the response of human T cells to an agonistic anti-CD27 mAb. In: AACR. 2012.
Disis ML, Patel MR, Pant S, Hamilton EP, Lockhart AC, Kelly K, et al. Avelumab (MSB0010718C; anti-PD-L1) in patients with recurrent/refractory ovarian cancer from the JAVELIN Solid Tumor phase Ib trial: safety and clinical activity. J Clin Oncol [Internet]. 2016;34(15_suppl):5533. https://doi.org/10.1200/JCO.2016.34.15_suppl.5533.
Kim K, Skora AD, Li Z, Liu Q, Tam AJ, Blosser RL, et al. Eradication of metastatic mouse cancers resistant to immune checkpoint blockade by suppression of. PNAS. 2014;111(32):1–6.
• Hamanishi J, Mandai M, Ikeda T, Minami M, Kawaguchi A. Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2015;33(34):11–3. Early trial detailing efficacy of checkpoint inhibition in ovarian cancer as a single agent.
Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2016;66(1):7–30. https://doi.org/10.3322/caac.21332.
Longoria TC, Eskander RN. Immunotherapy in endometrial cancer—an evolving therapeutic paradigm. Gynecol Oncol Res Pract [Internet]. 2015;11(2). Available from: doi: https://doi.org/10.1186/s40661-015-0020-3
Kondratiev S, Sabo E, Yakirevich E, Lavie O, Resnick MB. Intratumoral CD8+ T Lymphocytes as a prognostic factor of survival in endometrial carcinoma. 2004;10:4450–4456.
Ambros RA, Kurman RI. Combined assessment of vascular and myometrial invasion as a model to predict prognosis in stage I endometrioid adenocarcinoma of the uterine corpus. Cancer. 1992;69(6):1424–31. https://doi.org/10.1002/1097-0142(19920315)69:6<1424::AID-CNCR2820690620>3.0.CO;2-5.
Makker V, Filiaci VL, Chen L, Darus CJ, James E, Sutton G, et al. Phase II evaluation of dalantercept, a soluble recombinant activin receptor-like kinase 1 (ALK-1) receptor fusion protein, for the treatment of recurrent or persistent endometrial cancer: an NRG oncology/gynecologic oncology group study 0229N. Gynecol Oncol. 2015;138(1):24–9. https://doi.org/10.1016/j.ygyno.2015.04.006.
Alvarez EA, Brady WE, Walker JL, Rotmensch J, Zhou XC, Kendrick JE, et al. Phase II trial of combination bevacizumab and temsirolimus in the treatment of recurrent or persistent endometrial carcinoma: A Gynecologic Oncology Group study. Gynecol Oncol [Internet]. 2017;129(1):22–7. Available from:. https://doi.org/10.1016/j.ygyno.2012.12.022.
Powell MA, Sill MW, Goodfellow PJ, Doris M, Lankes HA, Leslie KK, et al. A phase II trial of brivanib in recurrent or persistent endometrial cancer: an NRG Oncology/Gynecologic Oncology Group study. Gynecol Oncol. 2014;135(1):38–43. https://doi.org/10.1016/j.ygyno.2014.07.083.
•• Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med [Internet]. 2015;372(26):2509–20. https://doi.org/10.1056/NEJMoa1500596. Clinical trial that catalyzed the study of checkpoint inhibitors in MMR-deficient endometrial cancer.
Goodfellow PJ, Billingsley CC, Lankes HA, Ali S, Cohn DE, Broaddus RJ, et al. Combined microsatellite instability, MLH1 methylation analysis, and immunohistochemistry for Lynch syndrome screening in endometrial cancers from GOG210: an NRG Oncology and Gynecologic Oncology Group study. J Clin Oncol. 2015;33(36):4301–8. https://doi.org/10.1200/JCO.2015.63.9518.
• McMeekin DS, Tritchler DL, Cohn DE, Mutch DG, Lankes HA, Geller MA, et al. Clinicopathologic significance of mismatch repair defects in endometrial cancer: an NRG Oncology/Gynecologic Oncology Group Study. J Clin Oncol [Internet]. 2016;34(25):3062–8. https://doi.org/10.1200/JCO.2016.67.8722. Study describing the frequency of MMR deficiency in endometrial cancer specimens.
Howitt BE, Shukla SA, Sholl LM, Ritterhouse LL, Watkins JC, Rodig S, et al. Association of polymerase e-mutated and microsatellite-instable endometrial cancers with neoantigen load, number of tumor-infiltrating lymphocytes, and expression of pd-2 and pd-l1. JAMA Oncol. 2015;1(9):1319–23. https://doi.org/10.1001/jamaoncol.2015.2151.
Ott PA, Bang Y, Berton-Rigaud D, Elez E, Pishvaian MJ, Rugo HS, et al. Safety and antitumor activity of pembrolizumab in advanced programmed death ligand 1-positive endometrial cancer: results from the KEYNOTE-028 study. J Clin Oncol [internet]. 2017;JCO2017725952. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28489510.
Chikuma S, Kanamori M, Mise-Omata S, Yoshimura A. Suppressors of cytokine signaling: potential immune checkpoint molecules for cancer immunotherapy. Cancer Sci. 2017;108(4):574–80. https://doi.org/10.1111/cas.13194.
Tewari KS, Sill MW, Long HJ, Penson RT, Huang H, Ramondetta LM, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med [Internet]. 2014;370(8):734–43. Available from:. https://doi.org/10.1056/NEJMoa1309748.
Yim E, Ph D, Park J, Ph D. The role of HPV E6 and E7 oncoproteins in HPV-associated cervical carcinogenesis. 2005;37(6):319–24.
Roszik, J, Qin, Y, Ekmekcioglu, S, Forget, M-A, Hwu, P, Grimm, E A., Jazaeri AA. The neoantigen landscape and immune regulators in cervical cancer. In: 2017 ASCO Annual Meeting. J Clin Oncol; 2017.
Lin, P Lin, Yen Y, Lee H. A combination of anti-PD-L1 mAb plus LM-LLO-E6 vaccine to supprss tumor growth and metastasis in HPV-infected cancers. In: 2017 ASCO Annual Meeting 2017.
Frenel, J-S, Le Tourneau, C, O’Neill, BH, Ott, PA, Piha-Paul, SA, Goomez-Roca, CA, Van Brummelen, EV, Rugo, HS, Thomas, S, Saraf, S, Chen, M, Varga A. Pembrolizumab in patients with advanced cervical squamous cell cancer: preliminary results from the phase IB Keynote-028 study. In: ASCO. 2016.
Schellens, Jan H.M., M Aurelien, Zeigenfuss, S, Ding, J, K P Scott, Chung HC. Pembrolizumab for previously treated advanced cervical squamous cell cancer: preliminary results from the phase 2 KEYNOTE-158 study. In: 2017 ASCO Annual Meeting. J Clin Oncol; 2017.
Zamarin D, Postow MA. Pharmacology & therapeutics immune checkpoint modulation: rational design of combination strategies. Pharmacol Ther [Internet]. 2015;150:23–32. Available from:. https://doi.org/10.1016/j.pharmthera.2015.01.003.
Naidoo J, Page DB, Li BT, Connell LC, Schindler K, Lacouture ME, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015;26(12):2375–91. https://doi.org/10.1093/annonc/mdv383.
Heery CR, O’Sullivan-Coyne G, Madan RA, Cordes L, Rajan A, Rauckhorst M, et al. Avelumab for metastatic or locally advanced previously treated solid tumours (JAVELIN solid tumor): a phase 1a, multicohort, dose-escalation trial. Lancet Oncol [Internet]. 2017;18(5):587–98. Available from:. https://doi.org/10.1016/S1470-2045(17)30239-5.
Weber JS, Yang JC, Atkins MB, Disis ML. Toxicities of immunotherapy for the practitioner. J Clin Oncol. 2015;33(18):2092–9. https://doi.org/10.1200/JCO.2014.60.0379.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare no conflicts of interest as they relate to the content of this manuscript. Ramez N. Eskander declares compensation for speaking on behalf of Genentech, AZ Oncology, and CLOVIS.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Gynecologic Oncology
Rights and permissions
About this article
Cite this article
Anderson, K., Eskander, R.N. Immune Checkpoint Inhibition in the Treatment of Gynecologic Cancer. Curr Obstet Gynecol Rep 7, 6–19 (2018). https://doi.org/10.1007/s13669-018-0231-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13669-018-0231-9