Abstract
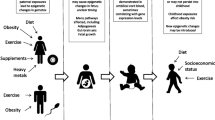
Epigenetic signatures, which can sometimes be transgenerationally inherited, include DNA methylation, histone covalent modifications, or chromatin folding, as well as microRNAs and other mechanisms that can regulate gene expression without altering the underlying genomic sequence. Maternal malnutrition during perinatal periods has been involved, through fetal or developmental programming, in the susceptibility to excessive adiposity and other non-communicable chronic diseases. Epigenetic encrypts in the adipose tissue of obese subjects, which are affected by nutrition, sedentarism, and age among other factors, can be also reflected in the blood cells. Relationships between obesity and the epigenetic regulation of gene expression have been repeatedly reported, for example. It has been suggested that the obesity status may be mediated by epigenetic marks and that the response to a hypocaloric diet could be related to the methylation profiles of specific gene promoters. This review is focused on the importance of epigenetic regulation, with emphasis on DNA methylation, in the etiology and development of obesity, presenting some target epiobesogenic genes that might be used as biomarkers of weight loss success and weight regain and for preventive and personalized nutrition.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Martinez JA, Navas-Carretero S, Saris WH, Astrup A. Personalized weight loss strategies-the role of macronutrient distribution. Nat Rev Endocrinol. 2014;10(12):749–60.
McAllister EJ, Dhurandhar NV, Keith SW, Aronne LJ, Barger J, Baskin M, et al. Ten putative contributors to the obesity epidemic. Crit Rev Food Sci Nutr. 2009;49(10):868–913.
Campión J, Milagro F, Martínez JA. Epigenetics and obesity. Prog Mol Biol Transl Sci. 2010;94:291–347.
Milagro FI, Campion J, Martinez JA. Handbook of epigenetics: the new molecular and medical genetics, Trygve Tollefsbol, editor. Elsevier, 2010.
Allis CD, Jenuwein T, Reinberg D. Epigenetics. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2007.
Vickers MH. Early life nutrition, epigenetics and programming of later life disease. Nutrients. 2014;6(6):2165–78.
Simmons R. Epigenetics and maternal nutrition: nature v. nurture. Proc Nutr Soc. 2011;70(1):73–81.
Soubry A, Hoyo C, Jirtle RL, Murphy SK. A paternal environmental legacy: evidence for epigenetic inheritance through the male germ line. Bioessays. 2014;36(4):359–71.
Gabory A, Roseboom TJ, Moore T, Moore LG, Junien C. Placental contribution to the origins of sexual dimorphism in health and diseases: sex chromosomes and epigenetics. Biol Sex Differ. 2013;4(1):5.
Zheng J, Xiao X, Zhang Q, Yu M. DNA methylation: the pivotal interaction between early-life nutrition and glucose metabolism in later life. Br J Nutr. 2014;112(11):1850–7.
Junien C, Gallou-Kabani C, Vigé A, Gross MS. Nutritional epigenomics of metabolic syndrome. Med Sci (Paris). 2005 Spec No: 44–52.
Barouki R, Gluckman PD, Grandjean P, Hanson M, Heindel JJ. Developmental origins of non-communicable disease: implications for research and public health. Environ Health. 2012;11:42.
Roadmap Epigenomics Consortium, Kundaje A, Meuleman W, Ernst J, Bilenky M, Yen A, et al. Integrative analysis of 111 reference human epigenomes. Nature. 2015;518(7539):317–30. This paper demonstrates the central role of epigenomic information for understanding gene regulation, cellular differentiation and human disease.
Leung D, Jung I, Rajagopal N, Schmitt A, Selvaraj S, Lee AY, et al. Integrative analysis of haplotype-resolved epigenomes across human tissues. Nature. 2015;518(7539):350–4. This paper enhances our understanding of the mechanisms by which cis-regulatory elements control gene expression programs.
Weber M, Hellmann I, Stadler MB, Ramos L, Paabo S, Rebhan M, et al. Distribution, silencing potential and evolutionary impact of promoter DNA methylation in the human genome. Nat Genet. 2007;39(4):457–66.
Kroeze LI, van der Reijden BA, Jansen JH. 5-Hydroxymethylcytosine: an epigenetic mark frequently deregulated in cancer. Biochim Biophys Acta. 2015;1855(2):144–54.
Schultz MD, He Y, Whitaker JW, Hariharan M, Mukamel EA, Leung D, et al. Human body epigenome maps reveal noncanonical DNA methylation variation. Nature. 2015. doi:10.1038/nature14465.
Fu Y, Dominissini D, Rechavi G, He C. Gene expression regulation mediated through reversible m6A RNA methylation. Nat Rev Genet. 2014;15(5):293–306.
Wang J, Wu Z, Li D, Li N, Dindot SV, Satterfield MC, et al. Nutrition, epigenetics, and metabolic syndrome. Antioxid Redox Signal. 2012;17(2):282–301.
Qureshi IA, Mehler MF. Developing epigenetic diagnostics and therapeutics for brain disorders. Trends Mol Med. 2013;19(12):732–41.
Holoch D, Moazed D. RNA-mediated epigenetic regulation of gene expression. Nat Rev Genet. 2015;16(2):71–84.
Puri V, Virbasius JV, Guilherme A, Czech MP. RNAi screens reveal novel metabolic regulators: RIP140, MAP4k4 and the lipid droplet associated fat specific protein (FSP) 27. Acta Physiol (Oxf). 2008;192(1):103–15.
Nolte-’t Hoen EN, Van Rooij E, Bushell M, Zhang CY, Dashwood R, James WP, et al. The role of microRNA in nutritional control. J Intern Med. 2015. doi:10.1111/joim.12372. This paper reviews the role of intracellular and extracellular miRNAs in nutritional control of various (patho)physiological processes.
Feng D, Lazar MA. Clocks, metabolism, and the epigenome. Mol Cell. 2012;47(2):158–67.
Scheen AJ, Junien C. Epigenetics, interface between environment and genes: role in complex diseases. Rev Med Liege. 2012;67(5–6):250–7.
Youngson NA, Morris MJ. What obesity research tells us about epigenetic mechanisms. Philos Trans R Soc Lond B Biol Sci. 2013;368(1609):20110337.
Laszlo AH, Derrington IM, Brinkerhoff H, Langford KW, Nova IC, Samson JM, et al. Detection and mapping of 5-methylcytosine and 5-hydroxymethylcytosine with nanopore MspA. Proc Natl Acad Sci U S A. 2013;110(47):18904–9.
Mansego ML, Milagro FI, Campión J, Martínez JA. Techniques of DNA methylation analysis with nutritional applications. J Nutrigenet Nutrigenomics. 2013;6(2):83–96. A large number of methods can be used for the analysis of DNA methylation such as pyrosequencing™, primer extension or real-time PCR methods, and genome-wide DNA methylation profile from microarray or sequencing-based methods.
Ong ML, Lin X, Holbrook JD. Measuring epigenetics as the mediator of gene/environment interactions in DOHaD. J Dev Orig Health Dis. 2015;6(1):10–6.
Kubota T, Miyake K, Hariya N, Mochizuki K. Understanding the epigenetics of neurodevelopmental disorders and DOHaD. J Dev Orig Health Dis. 2015;6(2):96–104.
Lukaszewski MA, Eberlé D, Vieau D, Breton C. Nutritional manipulations in the perinatal period program adipose tissue in offspring. Am J Physiol Endocrinol Metab. 2013;305(10):E1195–207.
Gluckman PD, Hanson MA, Low FM. The role of developmental plasticity and epigenetics in human health. Birth Defects Res C Embryo Today. 2011;93(1):12–8.
Rasmussen F, Johansson M. The relation of weight, length and ponderal index at birth to body mass index and overweight among 18-year-old males in Sweden. Eur J Epidemiol. 1998;14:373–80.
Adamo KB, Ferraro ZM, Brett KE. Can we modify the intrauterine environment to halt the intergenerational cycle of obesity? Int J Environ Res Public Health. 2012;9(4):1263–307.
Ganu RS, Harris RA, Collins K, Aagaard KM. Maternal diet: a modulator for epigenomic regulation during development in nonhuman primates and humans. Int J Obes Suppl. 2012;2 Suppl 2:S14–8.
Ruemmele FM, Garnier-Lengliné H. Why are genetics important for nutrition? Lessons from epigenetic research. Ann Nutr Metab. 2012;60 Suppl 3:38–43.
Waterland RA, Travisano M, Tahiliani KG, Rached MT, Mirza S. Methyl donor supplementation prevents transgenerational amplification of obesity. Int J Obes (Lond). 2008;32(9):1373–9.
Cordero P, Milagro FI, Campion J, Martinez JA. Maternal methyl donors supplementation during lactation prevents the hyperhomocysteinemia induced by a high-fat-sucrose intake by dams. Int J Mol Sci. 2013;14(12):24422–37.
Waterland RA. Early environmental effects on epigenetic regulation in humans. Epigenetics. 2009;4(8):523–5.
Szeto IM, Das PJ, Aziz A, Anderson GH. Multivitamin supplementation of Wistar rats during pregnancy accelerates the development of obesity in offspring fed an obesogenic diet. Int J Obes (Lond). 2009;33(3):364–72.
Yajnik CS, Deshpande SS, Jackson AA, Refsum H, Rao S, Fisher DJ, et al. Vitamin B12 and folate concentrations during pregnancy and insulin resistance in the offspring: the Pune Maternal Nutrition Study. Diabetologia. 2008;51(1):29–38.
Zhou D, Pan YX. Pathophysiological basis for compromised health beyond generations: role of maternal high-fat diet and low-grade chronic inflammation. J Nutr Biochem. 2015;26(1):1–8.
Levin BE, Dunn-Meynell AA. Maternal obesity alters adiposity and monoamine function in genetically predisposed offspring. Am J Physiol Regul Integr Comp Physiol. 2002;283(5):R1087–93.
Ojha S, Saroha V, Symonds ME, Budge H. Excess nutrient supply in early life and its later metabolic consequences. Clin Exp Pharmacol Physiol. 2013;40(11):817–23.
Heerwagen MJ, Miller MR, Barbour LA, Friedman JE. Maternal obesity and fetal metabolic programming: a fertile epigenetic soil. Am J Physiol Regul Integr Comp Physiol. 2010;299(3):R711–22.
Khalyfa A, Carreras A, Hakim F, Cunningham JM, Wang Y, Gozal D. Effects of late gestational high-fat diet on body weight, metabolic regulation and adipokine expression in offspring. Int J Obes (Lond). 2013;37(11):1481–9.
El Hajj N, Schneider E, Lehnen H, Haaf T. Epigenetics and life-long consequences of an adverse nutritional and diabetic intrauterine environment. Reproduction. 2014;148(6):R111–20.
Benyshek DC. The “early life” origins of obesity-related health disorders: new discoveries regarding the intergenerational transmission of developmentally programmed traits in the global cardiometabolic health crisis. Am J Phys Anthropol. 2013;152 Suppl 57:79–93.
Plagemann A. Perinatal nutrition and hormone-dependent programming of food intake. Horm Res. 2006;65 Suppl 3:83–9.
Lesage J, Sebaai N, Leonhardt M, Dutriez-Casteloot I, Breton C, Deloof S, et al. Perinatal maternal undernutrition programs the offspring hypothalamo-pituitary-adrenal (HPA) axis. Stress. 2006;9(4):183–98.
Wattez JS, Delahaye F, Lukaszewski MA, Risold PY, Eberlé D, Vieau D, et al. Perinatal nutrition programs the hypothalamic melanocortin system in offspring. Horm Metab Res. 2013;45(13):980–90.
Lesseur C, Armstrong DA, Paquette AG, Koestler DC, Padbury JF, Marsit CJ. Tissue-specific leptin promoter DNA methylation is associated with maternal and infant perinatal factors. Mol Cell Endocrinol. 2013;381(1–2):160–7.
Martínez JA, Cordero P, Campión J, Milagro FI. Interplay of early-life nutritional programming on obesity, inflammation and epigenetic outcomes. Proc Nutr Soc. 2012;71(2):276–83.
Saffery R. Epigenetic change as the major mediator of fetal programming in humans: are we there yet? Ann Nutr Metab. 2014;64(3–4):203–7.
Tarrade A, Panchenko P, Junien C, Gabory A. Placental contribution to nutritional programming of health and diseases: epigenetics and sexual dimorphism. J Exp Biol. 2015;218(Pt 1):50–8.
Milagro FI, Mansego ML, De Miguel C, Martínez JA. Dietary factors, epigenetic modifications and obesity outcomes: progresses and perspectives. Mol Aspects Med. 2013;34(4):782–812. Complex interactions among food components and histone modifications, DNA methylation, non-coding RNA expression and chromatin remodeling factors lead to a dynamic regulation of gene expression that controls the cellular phenotype.
Goni L, Milagro FI, Cuervo M, Martínez JA. Single-nucleotide polymorphisms and DNA methylation markers associated with central obesity and regulation of body weight. Nutr Rev. 2014;72(11):673–90. The aims of this review are to identify the single-nucleotide polymorphisms related to central obesity and to summarize the main findings on DNA methylation and obesity.
Campion J, Milagro FI, Martinez JA. Individuality and epigenetics in obesity. Obes Rev. 2009;10(4):383–92.
Marques-Rocha JL, Samblas M, Milagro FI, Bressan J, Martínez JA, Marti A. Non-coding RNAs, cytokines and inflammation-related diseases. FASEB J. 2015;29(9):3595–611.
Milagro FI, Miranda J, Portillo MP, Fernandez-Quintela A, Campión J, Martínez JA. High-throughput sequencing of microRNAs in peripheral blood mononuclear cells: identification of potential weight loss biomarkers. PLoS ONE. 2013;8(1):e54319. This research addresses the use of high-throughput sequencing technologies in the search for miRNA expression biomarkers in obesity. Basal expression of mir-935 and mir-4772, could be prognostic biomarkers and may forecast the response to a hypocaloric diet.
Ali O, Cerjak D, Kent Jr JW, James R, Blangero J, Carless MA, et al. An epigenetic map of age-associated autosomal loci in northern European families at high risk for the metabolic syndrome. Clin Epigenetics. 2015;7(1):12.
Drummond EM, Gibney ER. Epigenetic regulation in obesity. Curr Opin Clin Nutr Metab Care. 2013;16(4):392–7.
Berdasco M, Esteller M. Genetic syndromes caused by mutations in epigenetic genes. Hum Genet. 2013;132(4):359–83.
Okamura M, Inagaki T, Tanaka T, Sakai J. Role of histone methylation and demethylation in adipogenesis and obesity. Organogenesis. 2010;6(1):24–32.
Abete I, Navas-Carretero S, Marti A, Martinez JA. Nutrigenetics and nutrigenomics of caloric restriction. Prog Mol Biol Transl Sci. 2012;108:323–46.
Martin SL, Hardy TM, Tollefsbol TO. Medicinal chemistry of the epigenetic diet and caloric restriction. Curr Med Chem. 2013;20(32):4050–9.
Lomba A, Martínez JA, García-Díaz DF, Paternain L, Marti A, Campión J, et al. Weight gain induced by an isocaloric pair-fed high fat diet: a nutriepigenetic study on FASN and NDUFB6 gene promoters. Mol Genet Metab. 2010;101(2–3):273–8.
Uriarte G, Paternain L, Milagro FI, Martínez JA, Campion J. Shifting to a control diet after a high-fat, high-sucrose diet intake induces epigenetic changes in retroperitoneal adipocytes of Wistar rats. J Physiol Biochem. 2013;69(3):601–11.
Cordero P, Campion J, Milagro FI, Martinez JA. Transcriptomic and epigenetic changes in early liver steatosis associated to obesity: effect of dietary methyl donor supplementation. Mol Genet Metab. 2013;110(3):388–95.
Cordero P, Gomez-Uriz AM, Campion J, Milagro FI, Martinez JA. Dietary supplementation with methyl donors reduces fatty liver and modifies the fatty acid synthase DNA methylation profile in rats fed an obesogenic diet. Genes Nutr. 2013;8(1):105–13.
Boqué N, de la Iglesia R, de la Garza AL, Milagro FI, Olivares M, Bañuelos O, et al. Prevention of diet-induced obesity by apple polyphenols in Wistar rats through regulation of adipocyte gene expression and DNA methylation patterns. Mol Nutr Food Res. 2013;57(8):1473–8.
Paternain L, Batlle MA, De la Garza AL, Milagro FI, Martínez JA, Campión J. Transcriptomic and epigenetic changes in the hypothalamus are involved in an increased susceptibility to a high-fat-sucrose diet in prenatally stressed female rats. Neuroendocrinology. 2012;96(3):249–60.
Milagro FI, Gómez-Abellán P, Campión J, Martínez JA, Ordovás JM, et al. CLOCK, PER2 and BMAL1 DNA methylation: association with obesity and metabolic syndrome characteristics and monounsaturated fat intake. Chronobiol Int. 2012;29(9):1180–94.
Hermsdorff HH, Mansego ML, Campión J, Milagro FI, Zulet MA, Martínez JA. TNF-alpha promoter methylation in peripheral white blood cells: relationship with circulating TNFα, truncal fat and n-6 PUFA intake in young women. Cytokine. 2013;64(1):265–71.
do Amaral CL, Milagro FI, Curi R, Martínez JA. DNA methylation pattern in overweight women under an energy-restricted diet supplemented with fish oil. Biomed Res Int. 2014;2014:675021.
Gómez-Úriz AM, Milagro FI, Mansego ML, Cordero P, Abete I, De Arce A, et al. Obesity and ischemic stroke modulate the methylation levels of KCNQ1 in white blood cells. Hum Mol Genet. 2015;24(5):1432–40.
Gómez-Úriz AM, Goyenechea E, Campión J, de Arce A, Martinez MT, Puchau B, et al. Epigenetic patterns of two gene promoters (TNF-α and PON) in stroke considering obesity condition and dietary intake. J Physiol Biochem. 2014;70(2):603–14.
Campion J, Milagro FI, Goyenechea E, Martinez JA. TNF-alpha promoter methylation as a predictive biomarker for weight-loss response. Obesity (Silver Spring). 2009;17(6):1293–7.
Cordero P, Campion J, Milagro FI, Goyenechea E, Steemburgo T, Javierre BM, et al. Leptin and TNF-alpha promoter methylation levels measured by MSP could predict the response to a low-calorie diet. J Physiol Biochem. 2011;67(3):463–70.
Dahlman I, Sinha I, Gao H, Brodin D, Thorell A, Rydén M, et al. The fat cell epigenetic signature in post-obese women is characterized by global hypomethylation and differential DNA methylation of adipogenesis genes. Int J Obes (Lond). 2015. doi:10.1038/ijo.2015.31. Global CpG hypomethylation and over-representation of differentially methylated DNA sites in adipogenesis genes may contribute to adipose hyperplasia in post-obese women.
Perez-Cornago A, Mansego ML, Zulet MA, Martinez JA. DNA hypermethylation of the serotonin receptor type-2A gene is associated with a worse response to a weight loss intervention in subjects with metabolic syndrome. Nutrients. 2014;6(6):2387–403.
Lopez-Legarrea P, Mansego ML, Zulet MA, Martinez JA. SERPINE1, PAI-1 protein coding gene, methylation levels and epigenetic relationships with adiposity changes in obese subjects with metabolic syndrome features under dietary restriction. J Clin Biochem Nutr. 2013;53(3):139–44.
Milagro FI, Campión J, Cordero P, Goyenechea E, Gómez-Uriz AM, Abete I, et al. A dual epigenomic approach for the search of obesity biomarkers: DNA methylation in relation to diet-induced weight loss. FASEB J. 2011;25(4):1378–89.
Moleres A, Campión J, Milagro FI, Marcos A, Campoy C, Garagorri JM, et al. Differential DNA methylation patterns between high and low responders to a weight loss intervention in overweight or obese adolescents: the EVASYON study. FASEB J. 2013;27(6):2504–12. This article identified 5 DNA regions that are differentially methylated depending on weight loss response. These methylation changes may help to better understand the weight loss response in obese subjects.
Crujeiras AB, Campion J, Díaz-Lagares A, Milagro FI, Goyenechea E, Abete I, et al. Association of weight regain with specific methylation levels in the NPY and POMC promoters in leukocytes of obese men: a translational study. Regul Pept. 2013;186:1–6.
Lokk K, Modhukur V, Rajashekar B, Märtens K, Mägi R, Kolde R, et al. DNA methylome profiling of human tissues identifies global and tissue-specific methylation patterns. Genome Biol. 2014;15(4):r54. doi:10.1186/gb-2014-15-4-r54.
Acknowledgments
We thank the Government of Spain (MINECO, ref. AGL2013-45554-R), ISCII (CIBERobn and RETIC) and IdiSNA for financial support.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human and animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Diabetes and Obesity
Rights and permissions
About this article
Cite this article
Milagro, F.I., Riezu-Boj, J.I. & Martinez, J.A. Epigenetic Determinants of Weight Management: Methylation Signatures. Curr Nutr Rep 4, 330–339 (2015). https://doi.org/10.1007/s13668-015-0140-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13668-015-0140-8