Abstract
Gastrodia elata is a famous traditional Chinese herb with medicinal and edible application. In this study, three new polybenzyls, gastropolybenzylols G-I (1–3) were isolated from the EtOAc extract of G. elata. Their structures were identified by extensive spectroscopic analyses involving HRESIMS, UV, IR, 1D and 2D NMR. Compound 1 showed agonistic effects on MT1 and MT2 receptors with agonistic rates of 55.91±4.84% and 165.13±5.65% at the concentration of 0.5 mM, respectively, and an EC50 value of 76.24 μM on MT2 receptor.
Graphic Abstract

Similar content being viewed by others
1 Introduction
Gastrodia elata, a saprophytic herb from orchid family, mainly distributed in East Asia, Southeast Asia and Oceania. The main constituents of G. elata are phenolics, polysaccharides, sterols and organic acids [1]. As a famous traditional Chinese medicine (TCM), the tuber of G. elata is commonly used for the treatment of diverse mental diseases, involving insomnia [2, 3], depression [4,5,6,7], epilepsy, convulsion [8, 9], and Alzheimer’s disease [10, 11]. Plenty of pharmacological investigation suggested that G. elata had anti-hypertension [12], anti-tumor [13], anti-virus [14, 15] and anti-inflammation [16, 17] effects. Most of pharmacological components are 4-hydroxybenzyl alcohol, 4-hydroxybenzaldehyde, vanillin, 1,3-bis(4-hydroxybenzyl) citrate, 1-(4-beta-d-glucopyranosyloxybenzyl) citrate and parishin B [18]. However few reports about the psychoactive effects of polybenzyls were published [19,20,21,22]. In this study, the EtOAc extract of G. elata was found to activate MT2 receptor with an agonistic rate of 105.38% at the concentration of 102.22 μg/mL. In order to characterize the active constituents, three new compounds (1–3, Fig. 1) were obtained by various column chromatography. Herein, we report their isolation, structural elucidation and agonistic activities on melatonin (MT1 and MT2) receptors.
2 Results and Discussion
2.1 Structure Elucidation
Compound 1 had a molecular formula of C34H30O5 with 20 degrees of unsaturation, which was deduced from HRESIMS at m/z 517.2040 [M−H]− (calcd 517.2020). The UV absorptions at 281 and 254 nm, and the IR absorptions at 3426, 1631, 1613, 1511 and 1439 cm−1 suggested the presence of hydroxyl and phenyl groups. In the 1H-NMR spectrum, three sets of protons [δH 7.01 (4H, d, J = 8.3 Hz, H-2, H-6, H-2′‴, H-6′‴), 6.67 (4H, overlap, H-3, H-5, H-3′‴, H-5′‴); 6.93 (2H, d, J = 8.4 Hz, H-2‴, H-6‴), 6.67 (2H, overlap, H-3‴, H-5‴)], and combining with 1H-1H COSY correlations of H-2/H-3, H-6/H-5, H-2′‴/H-3′‴, H-6′‴/H-5′‴, and of H-2‴/H-3‴, H-6‴/H-5‴ indicated the existence of 1,4-substituted aromatic rings. One set of protons [δH 6.85 (1H, brs, H-6″), 6.84 (1H, J = 8.4, 2.0 Hz, H-4″), 6.67 (1H, overlap, H-3″)], and combining with 1H-1H COSY correlation of H-3″/H-4″ manifested the presence of a 1,2,4-substitued benzene ring. Besides, two meta-coupled protons [δH 6.77 (1H, d, J = 2.0 Hz, H-4′) and 6.76 (1H, d, J = 2.0 Hz, H-6′)], four methylenes [δH 3.79 (4H, s, H-7, H-7‴), 3.75 (2H, s, H-7′), 3.68 (2H, s, H-7″)] were well recognized (Table 1). Compared with 4-[[2-hydroxy-5-(methoxymethyl) phenyl] methyl]-2,6-bis [(4-hydroxyphenyl) methyl] phenol [19], compound 1 had an extra para- hydroxybenzyl [δH 6.93 (2H, d, J = 8.4 Hz, H-2‴, H-6‴), 6.67 (2H, overlap, H-3‴, H-5‴), 3.68 (2H, s, H-7″); δC 156.4 (C-4‴, s), 134.5 (C-1‴, s), 130.8 (C-2‴, C-6‴, d), 116.1 (C-3‴, C-5‴, d), 41.3 (C-7″, t)], but with the absence of a methoxymethyl group. Based on the HMBC correlations from H-7″ (δH 3.68, s) to C-2‴, C-6‴ (δC 132.1, d), C-4″ (128.5, d), C-6″ (δC 132.2, d), the linkages of C-7″ to C-5″ and C-1‴ were established (Fig. 2). Thus, compound 1 was elucidated and named as gastropolybenzylol G.
Compound 2 was assigned a molecular formula of C17H20O4 with 8 degrees of unsaturation according to the HRESIMS at m/z 333.1344 [M+HCOO]− (calcd for 333.1344). The UV (274, 247 and 226 nm) and the IR (3423, 1613 and 1513 cm−1) absorptions showed the presence of hydroxyl and phenyl groups. Compared with 4,4′-hydroxybenzyl ether [23], compound 2 had an additional ethoxymethoxyl group [δH 5.21 (2H, s, H-8′), 3.70 (2H, q, H-9′), 1.18 (3H, t, H-10′); δC 94.2 (C-8′, t), 65.2 (C-9′, t), 15.5 (C-10′, q)] in accordance with the HMBC correlation from H-8′ to C-9′. The HMBC correlation from H-8′ (δH 5.21, 2H, s) to C-4′ (δC 158.5, s) indicated the ethoxymethoxyl group at C-4′. Therefore, compound 2 was identified and named as gastropolybenzylol H.
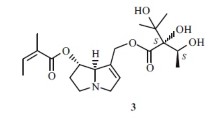
Compound 3 showed a molecular formula of C16H16O4 with 9 degrees of unsaturation according to the [M+H]+ ion at m/z 273.1122 (calcd for 273.1121). The UV absorptions at 275, 250 and 229 nm suggested the existence of phenolic structure and the IR absorptions at 3430, 1703, 1614 and 1518 cm−1 manifested the presence of hydroxyl, carbonyl and phenyl groups. Compared with 4,4′-hydroxybenzyl ether [23], compound 3 had an extra acetyl group [δC 172.9 (s, C-8′), 21.0 (q, C-9′)]. Thus, compound 3 was determined as the acetylated derivative of 4,4′-hydroxybenzyl ether and named as gastropolybenzylol I.
The known compounds were identified as 4, 4′-dimethoxy-dibenzyl ether [24] (4), 4,4′-diethoxybenzyl ether [25] (5), 4-[(4-(methoxymethyl) phenoxy] methyl] phenol [23] (6) and 4-[[4-[[4-(ethoxymethyl) phenoxy] methyl] phenoxy] methyl] phenol [26] (7).
2.2 MT1 and MT2 Receptors Agonistic Activities
Compounds 1 and 2 were evaluated for their agonistic activities on MT1 and MT2 receptors in vitro. Melatonin was used as the positive control. As showed in Fig. 3, compound 1 could activate MT1 and MT2 receptors with agonistic rates of 55.91±4.84 and 165.13±5.65% at the concentration of 0.5 mM. Due to 1 with better agonistic activities on MT2 receptor, further study provided an EC50 value of 76.24 μM on MT2 receptor (Fig. 4).
3 Experimental Section
3.1 General Procedures
NMR spectra were undertaken by Avance III-400/III-600 spectrometers (Bruker, Bremerhaven, Germany). UV spectra were recorded on a UV-2401 equipment (Shimadzu, Kyoto, Japan). IR spectra were obtained on Bio-Rad FTS-135 (Hercules, California, USA) spectrometers using KBr pellets. LCMS-IT-TOF (Shimadzu, Kyoto, Japan) was used for detecting MS spectra. Thin-layer chromatography (TLC) analyses were performed on silica gel GF254 plates (Jiangyou, Chemical Co. Ltd., Yantai, China). Compounds were purified by silica gel (200–300 mesh, Qingdao Makall group Co. Ltd., Qingdao, China), Sephadex LH-20 (Amersham Biosciences, Sweden) column chromatography and HPLC using Shimadzu LC-CBM-20 system (Shimadzu, Kyoto, Japan) with an Agilent XDB-C18 column (9.4 × 250 mm, Agilient, California, USA). 10% H2SO4 in EtOH was applied for detecting spots by heating after sprayed. Melatonin as positive control was obtained from Damas-beta Co. Ltd. (Shanghai, China).
3.2 Plant Material
Gastrodia elata Bl. was bought from Zhaotong in Yunnan Province of China, in September 2014, and was identified by Prof. Li-Gong Lei, Kunming Institute of Botany, CAS. A voucher specimen (No. 20141107) was deposited at the Laboratory of Anti-virus and Natural Medicinal Chemistry, Kunming Institute of Botany, CAS.
3.3 Extraction and Isolation
Fresh rhizomes of G. elata (45.0 kg) were cut into slices and extracted with 90% aqueous ethanol (45 L ×3) at room temperature. The combined extract was concentrated under reduced pressure and partitioned between H2O and EtOAc. The EtOAc extract (88 g) was separated by silica gel column chromatography (CC) using gradient elution with EtOAc-CHCl3 (0:100, 5:95, 10:90, 20:80, 40:60, 100:0, v/v) as the mobile phase to yield five fractions (Fr. 1–Fr. 5). Fr. 2 (22 g) was separated by silica gel CC with EtOAc-petroleum ether (5:95, 10:90, 20:80, 40:60, 100:0) to yield five fractions (Fr. 2.1–Fr. 2.5). Then, Fr. 2.2 (3.4 g) was further separated by silica gel CC (Me2CO-petroleum ether, 3:97, 5:95, 10:90, 20:80, 50:50) to give five fractions (Fr. 2.2.1–Fr. 2.2.5). Compounds 2 (10 mg) and 5 (30 mg) were purified from Fr. 2.2.1 by HPLC on an Agilent XDB-C18 column with the elution of MeCN–H2O (85:15, tR = 30 min) and MeCN-H2O (90:10, tR = 27 min), respectively. Compound 4 (24 mg) was isolated from Fr. 2.3 by Sephadex LH-20 (MeOH-CHCl3, 50:50) and finally purified by HPLC with the mobile phase of MeCN-H2O (60:40, tR = 23 min). Fr. 2.4 was purified from by Sephadex LH-20 (MeOH-CHCl3, 50:50) and HPLC on an Agilent XDB-C18 column with the elution of MeCN-H2O (60:40, tR = 20 min) to yield compound 3 (3.5 mg). Fr. 3 was subjected to silica gel CC (Me2CO-petroleum ether, 5:95, 10:90, 20:80, 40:60, 100:0) to yield five fractions (Fr. 3.1–Fr. 3.5). Fr 3.1 was isolated by silica gel CC (Me2CO-CHCl3, 5:95, 10:90, 20:80, 40:60, 100:0), Sephadex LH-20 CC (MeOH-CHCl3, 50:50), from which compounds 6 (2 mg, MeCN-H2O, 60:40, tR = 25 min) and 7 (37 mg, MeCN-H2O, 45:55, tR = 20 min) were obtained by HPLC purification. Further purification on Fr. 3.5 yielded compound 1 (10 mg) by silica gel CC, Sephadex LH-20 CC (MeOH-CHCl3 50:50) and semi-preparative HPLC (MeCN-H2O, 30:70, tR = 40 min).
Gastropolybenzylol G (1), pale yellow powder, UV (MeOH) λmax (log ε): 281 (4.03), 254 (3.25) nm; IR (KBr) νmax: 3426, 1631, 1613, 1511, 1439, 1247, 1229 cm−1; HRESIMS m/z 517.2040 [M−H]− (calcd 517.2020). 1H-NMR and 13C-NMR data showed in Table 1.
Gastropolybenzylol H (2), yellow colloidal solid, UV (MeOH) λmax (log ε): 275 (3.38), 248 (2.61), 226 (4.30) nm; IR (KBr) νmax: 3423, 1613, 1513, 1223, 1072, 1003 cm−1; HRESIMS m/z 333.1344 [M+COOH]− (calcd 333.1344). 1H-NMR and 13C-NMR data showed in Table 2.
Gastropolybenzylol I (3), white powder, UV (MeOH) λmax (log ε): 275 (3.48), 250 (2.89), 229 (4.42) nm; IR (KBr) νmax: 3430, 1703, 1614, 1518, 1268, 1245, 1029, 830 cm−1; HRESIMS m/z 273.1117 [M+H]+ (calcd 273.1121). 1H-NMR and 13C-NMR data showed in Table 2.
3.4 Bioassay of Agonistic Activities on MT1 and MT2 Receptors
Agonistic activities of compounds 1 and 2 were evaluated on HEK293 cell lines stably expressing the human melatonin MT1 and MT2 receptors. Referring to the previous study [27, 28], cells were cultivated by adding Dulbecco’s modified eagle medium, G418 (400 μg/mL) and fetal bovine serum (10%) in 5% CO2 incubator at 37 °C. Then, cells were put in a Matrigel® coated 96-well black plate at a density of 4 × 104/well and proliferated in CO2 for 24 h. Wash Free Fluo-8 Calcium Assay Kit (HD Biosicences Co. Ltd., Hd03-0010) was used for detecting calcium flow assay. Flex Station 3 Benchtop Multi-Mode Micro Plate Reader was applied for recording and reading data at room temperature using specified settings (excitation wave length, 485 nm, emission wave length, 525 nm, emission cut-off, 515 nm). EC50 values were calculated by GraphPad Prism 5 software.
References
L.M. Ojemann, W.L. Nelson, D.S. Shin, A.O. Rowe, R. Buchanan, Epilepsy Behav. 8, 376–383 (2006)
Y. Zhang, M. Li, R. Kang, J. Shi, G. Liu, J. Zhang, Pharmacol. Biochem. Behav. 102, 450–457 (2012)
Y. Shi, J.W. Dong, L.N. Tang, R.X. Kang, J.G. Shi, J.J. Zhang, Pharmacol. Biochem. Behav. 126, 146–151 (2014)
J.W. Jung, B.H. Yoon, H.R. Oh, J.H. Ahn, S.Y. Kim, S.Y. Park, J.F. Ryu, Biol. Pharm. Bull. 29, 261–265 (2006)
B.H. Zhou, X.J. Li, M. Liu, Z. Wu, X.M. Hu, Fitoterapia 77, 592–594 (2006)
S. Lin, W. Chen, K. Lu, P. Chen, S. Hsieh, T. Pan, S. Chen, L. Sheen, J. Agric. Food Chem. 62, 10493–10503 (2014)
P. Chen, C. Hsieh, K. Su, Y. Hou, H. Chiang, L. Sheen, Am. J. Chin. Med. 37, 1113–1124 (2009)
C. Hsieh, J. Lin, S. Chiang, S. Su, N. Tang, G. Lin, I. Lin, C. Liu, C. Hsiang, J. Chen, T. Ho, J. Ethnopharmacol. 109, 241–247 (2007)
J.H. Ha, D.U. Lee, J.T. Lee, J.S. Kim, C.S. Yong, J.A. Kim, J.S. Ha, K. Huh, J. Ethnopharmacol. 73, 329–333 (2000)
D.S.H.L. Kim, J. Kim, Y.S. Han, J. Altern. Complem. Med. 13, 333–340 (2007)
Y. Hu, C. Li, W. Shen, Neuropathology 34, 370–377 (2014)
O. Lee, K. Kim, C. Han, Y. Kim, H. Hong, Int. J. Mol. Sci. 13, 698–709 (2012)
J. Heo, S. Woo, M. Son, J. Park, W. Choi, K. Chang, S. Kim, E. Yoon, Y. Kim, H.M. Shin, S. Lee, Oncol. Rep. 18, 849–853 (2007)
H. Qiu, W. Tang, X. Tong, K. Ding, J. Zuo, Carbohydr. Res. 342, 2230–2236 (2007)
X. Tong, H. Qiu, X. Zhang, L. Shi, G. Wang, F. Ji, H. Ding, W. Tang, K. Ding, J. Zuo, Acta Pharmacol. Sin. 31, 585–592 (2010)
J.Y. Lee, Y.W. Jang, H.S. Kang, H. Moon, S.S. Sim, C.J. Kim, Arch. Pharm. Res. 29, 849–858 (2006)
S.M. Hwang, Y.J. Lee, D.G. Kang, H.S. Lee, Am. J. Chin. Med. 37, 395–406 (2009)
X.D. Yang, J. Zhu, R. Yang, J.P. Liu, L. Li, H.B. Zhang, Nat. Prod. Res. 21, 180–186 (2007)
.G. Shi, N.H. Chen, Y.N. Wang, Y.H. Yuan, S. Lin, M.M. Wu, Q.L. Guo, C.G. Zhu, China Patent CN103420805A, Dec 2013
J.Y. Huang, Y.H. Yuan, J.Q. Yan, Y.N. Wang, S.F. Chu, C.G. Zhu, Q.L. Guo, S.J. Gong, N.H. Chen, Acta Pharmacol. Sin. 37, 74–83 (2016)
X.L. Zhang, Y.H. Yuan, Q.H. Shao, Z.Z. Wang, C.G. Zhu, J.G. Shi, K.L. Ma, X. Yan, N.H. Chen, Toxicol. Lett. 271, 74–83 (2017)
Q.H. Shao, X.L. Zhang, Y. Chen, C.G. Zhu, J.G. Shi, Y.H. Yuan, N.H. Chen, Mol. Immunol. 99, 115–123 (2018)
H. Taguchi, Y. Heihachiro, Y. Itiro, K. Yamasaki, I.H. Kim, Chem. Pharm. Bull. 29, 55–62 (1981)
S.G. Kim, K.N. Chung, S.B. Yang, J. Org. Chem. 52, 3917–3919 (1987)
J. Zhou, X.Y. Pu, Y.B. Yang, Sci. Bull. 18, 1118–1120 (1981)
Z.F. Li, Y.W. Wang, H. Ouyang, Y. Lu, Y. Qiu, Y. Feng, H. Jiang, X. Zhou, S. Yang, J. Chromatogr. B 988, 45–52 (2015)
Y.L. Zhang, L.H. Lai, S.M. Wang, Chin. J. Biotechnol. 25, 457–463 (2009)
Y. Hasegawa, J.R. Erickson, G.J. Goddard, S.X. Yu, S.Y. Liu, K.W. Cheng, A. Eder, K. Bandoh, J. Aoki, R. Jarosz, A.D. Schrier, K.R. Lynch, G.B. Mills, X.J. Fang, J. Biol. Chem. 278, 11962–11969 (2003)
Acknowledgements
This work was supported by the Program of Yunling Scholar, the Youth Innovation Promotion Association, CAS (2013252), and the Applied Basic Research Programs of Yunnan Province (2017FB137).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
These authors have no conflict of interest to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Chen, SY., Geng, CA., Ma, YB. et al. Melatonin Receptors Agonistic Activities of Phenols from Gastrodia elata. Nat. Prod. Bioprospect. 9, 297–302 (2019). https://doi.org/10.1007/s13659-019-0213-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13659-019-0213-2