Abstract
Key message
Comparisons between compression and opposite wood formation in prostrating Pinus mugo indicate that the secondary meristem can produce more tracheids with thicker walls by also increasing the number of contemporaneously differentiating cells, rather than only increasing the duration or the rate of cell formation.
Context
Although cambium tissues within a stem experience the same climatic conditions, the resulting wood structure and properties can strongly differ. Assessing how meristem differently regulates wood formation to achieve different anatomical properties can help understanding the mechanisms of response and their plasticity.
Aims
We monitored the formation of compression (CW) and opposite (OW) wood within the same stems to understand whether achieved differences in wood structure are caused by modifications in the process of cell formation.
Methods
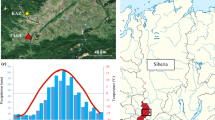
We collected weekly microcores of compression and opposite wood from the curved stem of ten treeline prostrating mountain pines (Pinus mugo Turra ssp. mugo) at the Majella massif in Central Italy.
Results
Results indicate that cambium formed approximately 1.5 times more cells in CW than OW, despite that CW cell differentiation only extended 2 weeks longer and the residence time of CW cells in the wall-thickening phase was only 20% longer. Differences in their formation were thus mainly related to both the rates and the width of the enlarging and wall-thickening zones (i.e., the number of cells simultaneously under differentiation) and less to duration of cell formation.
Conclusion
We conclude that to achieve such a different wood structures, the efficiency of the secondary meristem, in addition of altered rate of cell division and differentiation, can also modify the width of the developing zones. Thus, deciphering what rules this width is important to link environmental conditions with productivity.
Similar content being viewed by others
1 Introduction
Trees and shrubs growing in forests characterized by temperate climates form one growth ring every year. In such environments, temperature and water availability are often the most important factors controlling tree growth (e.g., Körner 2006), and cambium is usually active from spring to fall (Rathgeber et al. 2016). This period, in which wood formation occurs, is also the time window when xylem differentiates. Environmental conditions prior to or during this time window directly affect the number and properties of the cells formed (Castagneri et al. 2017; Frankenstein et al. 2005; Fonti et al. 2010) and thus the functioning of the xylem. Inversely, several tree-ring characteristics can be used as proxy to reconstruct past environmental conditions (e.g., Solomon et al. 2007). Due to this crucial role in linking environment to tree functioning and performance, studying the patterns of xylem differentiation and the dynamics of tree growth in the past has become a major ecophysiological challenge to predict global warming scenario (e.g., Cailleret et al. 2017; Rossi et al. 2016).
Wood formation, although potentially influenced during every phase of development, usually responds to the environment mainly by modifying the timing and rates of cell formation (e.g., Cuny et al. 2012, 2014). These modifications induced by changing environmental conditions allow matching wood production with resources availability. In Mediterranean mountains, this timeframe of optimum environmental conditions is particularly constrained by both low temperature in winter and drought in summer. Therefore, xylogenesis studies, i.e., continuous cytological observations of forming tree rings (e.g., Gričar and Čufar 2008; Rossi et al. 2008b), performed in erratic Mediterranean climatic conditions, can be of particular help to better understand how the environment constrains growth processes and eventually impacts the wood structure (Fonti and Jansen 2012).
There are cases, however, where wood cells from the same meristem can have different characteristics despite being formed under the same environmental conditions. For example, mechanical stress, such as snow pressure acting on a tree stem (Groover 2016), may generate differing growth responses within the same tree. These differing responses are aimed at producing wooden tissues characterized by different properties such as the reaction wood, which is produced for re-equilibrating the static balance of the tree by locally modifying its xylem structure (e.g., Heinrich and Gärtner 2008). In most gymnosperms, this tissue is called compression wood (CW), since it is formed on the lower side of branches and leaning stems and exerts a high compressive growth stress on the xylem surface in the compression wood region (Yamamoto et al. 1991; Yamashita et al. 2007). Compression wood is typically characterized by larger annual rings with higher density mainly associated to changes in the number of the wooden cells and their anatomical-chemical characteristics. Indeed, the tracheids forming the reaction wood are usually shorter, display a more rounded cross-section with intracellular spaces (Timell 1986a, b), and have higher amounts of lignin and galactan and less cellulose and galactoglucomannan in their cell wall (Donaldson 2001; Nanayakkara et al. 2005, 2009; Timell 1986b). Moreover, the cell wall, in comparison with the normal wood, is thicker and characterized by the absence of the S3 cell wall layer, has a thickened lignification of the S2 layer (Côté et al. 1968; Donaldson 2001; Donaldson et al. 1999; Singh and Donaldson 1999), and displays helical cavities and higher microfibril angle (Donaldson et al. 2004).
Since CW displays excellent physical, structural, and mechanical characteristics (Gardiner and Macdonald 2005), this wood is very important for the 3- to 5-m-tall and multi-stemmed dwarf mountain pine (Pinus mugo Turra spp. mugo) growing at the treeline ecotone of the Majella massif (Majella National Park, Italy), where it is regularly prostrate and forms the «krummholz mat» [sensu Lehner and Lütz 2003]. Indeed, this species can survive such a harsh environment also because it has very flexible stems able to re-establish their vertical position every spring after snow melting. Trees regain their vertical orientation thanks to the formation of compression wood induced by the generation of compressive growth stress on the compression side of the lying stems. Despite that the growing conditions ruling the development of wood tissues within the same individual are usually the same, there are clear evidences that the process of wood formation can locally substantially differ to obtain the required structural characteristics. For example, the tapering of conduits observed along the tree stem, which is an important wood anatomical feature to reduce hydraulic resistance in woody plants (Anfodillo et al. 2012), has been linked to differences in duration of cell expansion.
In this study, we monitored wood formation to assess how the meristem, when operating within the same tree and under the same environmental conditions with the only exception of being on the compression side of a lying stem, can differently regulate its development to achieve very different structural properties. We expected to gain deeper insights into the processes that determine the structural characteristics of the wood and that can be relevant to better understand the mechanisms of cambial responses and their range of plasticity. Therefore, we compared the characteristics of wood formation focusing on the CW and OW tissues within the stem of mountain pine trees undergoing repeated mechanical stress, as the ones at the Majella massif in Central Italy.
2 Materials and methods
2.1 Study site
The research area is situated at the southern limit of the mountain pine krummholz distribution. The study site (42° 8′ 50″ N, 14° 6′ 18″E—Fig. 1) consists in a plot of about 1.5 ha, which is located at 1900 m a.s.l. at the subalpine-alpine ecotone of the State Natural Reserve Valle dell’Orfento, within the Majella National Park. In this area, the dense and close mountain pine vegetation borders with the adjacent beech forest at its altitudinal limit (timberline). The climate is Mediterranean, but the site being at high elevation experiences more continental conditions than at lower elevation. The approximately 6-km close meteorological station of Sant’Eufemia a Majella (888 m a.s.l.) recorded a mean annual temperature of 10.7 °C and annual total precipitation of 1430 mm, mainly concentrated in autumn and winter (data for the period 1932–2009).
Location and sampling design. Up-left: location of the study area in the Majella National Park, Abruzzo region—Italy. Up-right: the plot (red square) is located just above the upper limit of the beech forest (Fagus sylvatica L.). Bottom-left: example of sampled individuals. Bottom-right: schema indicating the stem sampling location for both the compression wood (CW) and opposite wood (OW)
2.2 Microcore sampling and processing
Sampling was performed from 6 May to 6 October 2011. Specifically, every week, ten new mountain pine stems with clear “krummholz” characteristics, i.e., a pronounced curvature at the stem basis, were selected for the extraction of two microcores (1.8 mm in diameter, 15 mm in length) containing the bark, cambium, and the last formed tree-rings using a Trephor (Rossi et al. 2006a). Weekly tree selection occurred by selecting the first ten individuals displaying a large stems with obvious stem curvature within a ~ 10-m2 sector randomly positioned within the pine forest. Microcores were collected from both the compression wood (CW, the stem side oriented downhill) and the opposite wood (OW, the uphill side of the stem) close to the tree stem base, where the curvature usually is the most pronounced. We selected ten new individuals every week because of the generally small stem diameter (5–10 cm), to exclude the risk of taking samples affected by wound effects caused by previous samplings. We sampled 210 trees collecting a total of 420 microcores (two microcores per tree) over the whole growing season. Immediately after the sampling, the microcores were deposited in Eppendorf vials with 75% ethanol and 25% acetic acid for 24 h and then stored in 70% alcohol solution to preserve the forming cells from degradation. Successively, microsections were prepared for cellular analysis following the procedure for wood microsections described in Schweingruber (2006). The procedure consisted in cutting 10–12-μm-thick transversal microsections with a sliding microtome and to stain them with safranin and astrablue, before fixation in Canada balsam.
2.3 Identifying compression wood
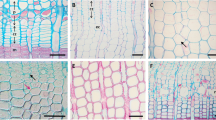
For CW microsections, the severity of compression of all the growing rings included in the microcores was additionally assessed according to the classification of Yumoto et al. (1983) (see Fig. 2) to verify the presence and intensity of compression in those that were taken as CW sampling. Observations of severity were performed on the microsections with a light microscope with a 400× magnification. The number of cells of the ring formed in the year 2010, 1 year before our sampling, was also counted in order to better evaluate the differences in number of cells between CW and OW.
Images indicating compression wood severity index, classified according to the grading system of Yumoto et al. (1983) and revised from Timell (1986a, b). A = very faint round cells; B = slightly round cells and slightly thicker cell walls; C = fairly round cells with thicker cell walls; D = round cells with some intercellular spaces and relatively thick cell walls; I = round but not fully circular cell with intercellular space and thick cell walls; S = nearly circular cells with intercellular space and very thick cell walls
2.4 Quantifying xylem formation
Ring formation was assessed by cellular observations with an Olympus BX 40 light microscope at a 400× magnification. Cellular observations consisted in counting the number of forming tracheids in the cambial, enlarging (E), wall thickening (W), and maturing (M) zones along three radial files on each microsection (Rossi et al. 2006b). The assignment of each cell to the different developmental stages was performed based on visual criteria. Cells of the cambial zone are typically flattened, flexible with only very thin, and not lignified primary wall. An increase in the number of cells in the cambial zone was used as an indicator for the start of cambial activity. Enlarging cells (E) are characterized by thin primary cell walls, with irregular radial diameter, roughly at least twice those of the dividing cambial cells. Wall-thickening (WT) cells were distinguished from enlarging cells by polarized light, because of the birefringence of the secondary cell wall. The end of wall thickening corresponds to the complete lignification of xylem cells; fully mature tracheids (M) were recognized by red-stained (by safranin) cell walls and empty lumina. The first appearance of cells in the enlargement phase was used to determine the onset of radial stem growth. When all tracheids of the developing ring reached maturity, radial stem growth was considered finished. In order to achieve higher robustness of the results by reducing variability in growth rates, at each sampling date, we excluded the two trees displaying the highest and lowest number of cells in the 2010 annual ring. Data fitting and all the relative statistical analyses were performed for each wood type using DOY-based average data. Means were obtained by first averaging cell counts of three radial cell files on each microsection, and subsequently by averaging the counting for the eight selected trees. The average weekly count of cells in each differentiation phase was then expressed as a function of the DOY according to the principles described in Rathgeber (2012).
2.5 Assessing timing and duration
The R (R Development Core Team 2007) package mgcv (Wood 2017) and scam (Pya and Wood 2015) were used for fitting generalized and shape-constrained additive model (gam and scam) to the number of cells in the different phenological phases to assess the timing and duration of cell formation, similarly to Cuny et al. (2013). First, fitting of the average number of cells in each phase (number of enlarging, wall thickening) along the DOY was done using gam function (with family quasi Poisson and with the parameters gamma = 1.4 and min.sp. = 0.001 and 0.025 for enlarging and wall thickening, respectively), while the fitting for the total number of cells (enlarging + wall thickening + mature cells) was achieved using the monotonic scam (k = − 1, bs = “mpi”, m = 2, gamma = 1.4, family = gaussian). Prior to fitting, the number of mature cells was kept stable after the last enlarging cells (DOYendE) at a value corresponding to the median of observed mature cell after DOYendE. Second, a plot showing the “dynamic of wood formation” was created by plotting three fitted curves, specifically:
where nE(t), nW(t), and nM(t) are the number of cells in the enlargement, thickening, and mature zones at time t.
The calculation of the cell timing was then derived from the plotted curves as the difference between the curves along the horizontal axis. For each type of wood (CW and OW), we took the average cell number predicted by the fitted gam and scam to calculate the timing (date of onset and cessation) and duration of residence that each tracheid spent in both the enlarging and wall-thickening phase (Cuny et al. 2013).
3 Results
3.1 Number and characteristics of cell produced
The number of tracheids produced during the growing season 2010 (the year prior the monitoring study) strongly differed among the 210 mountain pine stems. The number of cells counted ranged from 14 to 200 in CW and from 4 to 133 in OW. In 74% of the analyzed growing rings, we observed a higher number of tracheids in CW than in OW, where 37% and 17% of them exceeded, respectively, 1.5 and 2.0 times the number of tracheids in OW (Fig. 3). According to the classification of the compression wood severity index (Fig. 2), 79 stems (38%) were characterized by marked presence of compression wood with thick secondary wall (grade D, I, and S), and 80% of which had a number of cells higher than the mean value (n = 64). During the 2011 growing season, the proportion of stem with marked presence of compression wood increased substantially: out of the 140 stems with mature tracheids (i.e., collected after DOY 152), 67% were classified in the severity classes between D, I, and S, while the others were assigned to less severe classes, B to C. In contrast, we never detected presence of compression wood in the OW samples.
3.2 Timing of cambial phenology
The observations completed on the collected microcores (Fig. 4) indicated that cambial division occurred between the beginning of May and the beginning of October. At the first two sampling dates, on 6 May and 12 May (DOY 126 and 132), the cambial zone exhibited an increased number of dividing cells (9 to 12 cells instead of the 4 to 7 usually observed in the dormant season, i.e., at the end of our sampling campaign) indicating that cambial division already started, irrespective of the type of wood (Fig. 4a) and remained active, until the end of June, with a continuing decreasing number of dividing cells in CW after DOY 200 (July 19th).
Boxplot comparing of number of cells counted at each DOY in both the forming opposite (OW, blue) and the compression wood (CW, red) of each individual (n = 8 individuals in each group). From top to bottom, the counted cells in the cambial (C), enlarging (E), the secondary wall thickening (W), and the mature zones (M) are indicated. Lines indicate loess smoothings with confidence interval (loess smoother span = 0.4)
The first enlarging cells were observed for both types of wood on DOY 140 (May 20th), with a mean number of 3.57 ± 1.04 cells in OW and 4.50 ± 1.71 cells in CW (Fig. 4b). The production of enlarging cells reached a maximum around the middle of June, when both types of wood showed about the same amount of enlarging cells (6.9 ± 1.6 in OW and 7.1 ± 1.6 in CW, Fig. 5). A second maximum in the number of enlarging cells appeared after DOY 203 (July 23rd). The enlarging phase ended around the beginning of September, about 10 days earlier in OW than in CW (on DOY 241 and 251, respectively).
Dotplot indicating the difference in counted number of cells among pairs within each individual (n = 8 at each DOY). From top to bottom, the counted cells in the cambial (C), enlarging (E), the secondary wall thickening (W), and the mature zones (M) are indicated. Lines indicate loess smoothings with confidence interval (smoother span = 0.4)
Similarly as in the enlarging cells, both in OW and CW earlywood tracheids, the thickening of walls started at the same time on 1 June (DOY 152), whereas they ended at different times (Fig. 4c). The last differentiation cells of CW were observed on 6 October (DOY 279), 2 weeks later than for OW (September 22nd, DOY 265, Figs. 4d and 6a). Here, strong differences were also observed regarding the number of cells (Fig. 5). The number of observed wall-thickening tracheids at the first observation was already about 3 times higher in CW (4.1 ± 1.97) than in OW (1.43 ± 1.07) (Fig. 4c). The difference increased to 10–15 cells and kept constant until DOY 251, before decreasing. As a result of the process of wood formation, the 2011 growing period was 15 days longer in CW (about 140 days) than in OW (about 125 days, Fig. 6a). First, mature cells appeared at DOY 168 (Fig. 4d). The increased number of dividing cambial cells on CW appeared to occur in two periods and slight slowed around DOY 180–200, likely between the earlywood and latewood cell formation (Fig. 5a). After DOY 258 (September 15th), no new cells were built and the difference in number of mature cells between CW and OW stabilized at around 20–25 cells (Fig. 5d). This indicates that the meristem generating CW produced on average about 1.5 more cells than OW.
Timing of cell formation. a Plot showing the dynamic of wood formation for each type of wood (red = compression wood, blue = opposite wood). Each rectangular indicates the timing of the duration the cells have spent in the enlargement (light gray filling) and wall-thickening phase (gray). Dotted vertical lines indicate the onset and cessation of the growing season based on direct observation (see Fig. 3). b Comparison of cell residence time in enlarging and wall-thickening phase for each type of wood. The position on the x-axis is defined as the timing the cell enters in the corresponding phase. Vertical lines indicate half of the total number of cells
3.3 Duration of tracheid formation
The observation in timing and duration each tracheid spent into the enlarging and wall-thickening phases (Fig. 6a) confirms that the differences between the formation of OW and CW were mainly related to the tracheid residence time in the wall-thickening phase. On average, the CW cells spent 3 days less in the enlarging phase than the OW cells (6 and 9 days, respectively), though resided 4 days longer in the phase of wall thickening (23 and 19 days, respectively) (see also Table 1 for more detailed description for early and late formed cells). These differences varied along the season, with generally more time spent in the enlarging phase for the earlywood cells and more time in the wall-thickening phase for the latewood cells, with inverted differences around DOY 170 and 200 for the enlarging and wall-thickening phase, respectively (Fig. 6b).
4 Discussion
Studies on the dynamics of wood formation are usually performed to better understand how the environment affects wood structure, and thus, many studies focused on comparison between species (Rossi et al. 2008a) or along elevation (Moser et al. 2010; Prislan et al. 2011), or were conducted in experimental settings by controlling temperature (Fonti et al. 2013; Gričar et al. 2006, 2007) or moisture (Eilmann et al. 2009; Galle et al. 2010). These studies helped to quantify the plasticity of wood formation (Deslauriers et al. 2017) and showed how environmental conditions impact physiological processes (i.e., the initiation, termination, rate, and extent of growth, Lempereur et al. 2015) and the resulting anatomical structure (Fonti et al. 2010). Such studies also confirmed that temperature usually induces the onset of growing season and thus directly influences the amount of wood annually produced (Cocozza et al. 2016; Rossi et al. 2010). In contrast, in this study, we compared the formation of two very different types of wood (i.e., compression and the opposite wood), occurring within the same annual growth ring and produced under the same climatic conditions, to assess variability in xylem phenology and growth dynamics of mountain pine at a Mediterranean treeline. Since higher content of compression wood in high-altitude trees indicates the response to regular disturbing events (strong wind, heavy snow), and because many wood traits are correlated with growth rates and competition levels, it is expected that insights on CW and OW xylogenesis of these mountain pines can provide a better understanding of the impact of environmental conditions on forest health and productivity.
Our study confirms that, in this environment, the growth rings of mountain pines displayed a notable eccentricity to re-equilibrate stem static and regain its up-right standing position. In particular, the CW was approx. 1.5 times wider than OW, and with structural changes mainly occurring at the level of number and anatomical properties of the latewood cells. These observations also indicated that about 2/3 of the microcores collected from the CW were, indeed, characterized by a high severity index (class S, I, or D), i.e., by tracheids with thicker and denser walls. Finally, despite the wide differences in growth rate (and number of counted cells) among individual trees, due to limited stem size impeding repeated sampling on the same trees, the variability in number of cells between the groups resulted to be fairly similar and equally represented (i.e., each observation on CW was paired with an observation on OW within the same tree) allowing (noisier but) unbiased comparisons.
The results obtained showed that, despite the notable CW-induced growth eccentricity, the growing season of mountain pines at the Majella treeline displays a comparable timing and duration than other conifer species at colder ecosystems in the Northern Hemisphere (Rossi et al. 2016). The duration of the growing season (from end of May to beginning of October) was instead about 1 month longer than at the Alpine treeline, as monitored for Larix decidua Mill. and Picea abies (L.) (Moser et al. 2010; Rossi et al. 2008b).
Our study showed that the phases of cell enlarging and wall thickening started at the same time in the growing season for both CW and OW, despite the different number of cells under formation,. Nevertheless, the cessation in CW was 2 weeks later than in OW. This extension slightly confirms previous reports that high number of dividing cells increases the time of cell differentiation and maturation (Rossi et al. 2012), although the extension is minimal compared to the large difference in number of produced cells. It is, however, expectable that an extended growing period of CW, due to later growth cessation, can expose trees to a premature halt of latewood CW cells due to rapid worsening in weather conditions, which may induce the formation of “blue rings” (see Piermattei et al. 2015), as observed in 2009 for 13% of the sampled individuals (Supplementary Material). However, considering that 2 weeks represent a relatively short time to alone explain the difference in the achieved number of cells, we suggest that these differences in the number and structure of the produced cells are not solely related to an extended duration of the growing season.
The time each cell spent in the phase of enlargement and wall thickening (Fig. 6) showed that CW cells were also not residing longer enough (i.e., only 4–5 days or 20% longer residence time in the wall-thickening zone than OW cells) to explain their increased wall thickness. In total, the average time for a cell to be formed (including enlarging and wall-thickening phases) was approximately the same for both wood types (29 and 28 days for CW and OW, respectively). This implies that the enhanced wall thickening of the CW tracheids has to be related also to an increased wall deposition rate. However, considering that the number of cells to be differentiated is 1.5 higher than for the OW, the rate of wall thickening of CW’s tracheids should be extremely fast to process more numerous and significantly thicker wall cells with only few additional days. We, therefore, advocate that the increased efficiency in differentiating more numerous and thicker cells of the CW meristem is also achieved by differentiating more cells at the same time, i.e., by forming a wider band of enlarging and wall-thickening cells that are processed at the same time. Indeed, CW was almost constantly having a higher number of cells in the wall-thickening zones (Fig. 5d). These comparisons of wood formation between pairs, thus, support the findings that the two types of wood differ in their structure, especially considering the characteristics of the latewood, which is thicker and characterized by a higher density (Timell 1973).
This strategy of modifying the radial width of the forming zones, rather than radically changing rates and durations of forming tracheids, may imply a different perspective on the role of hormone concentrations in the regulation of the secondary meristem in this conifer. Indeed, the concentration of auxins along the stem seemingly controls the rate of conduit differentiation and size and the amount of lignin (Aloni and Zimmermann 1983). The formation of CW obviously requires that the meristem is supplied by higher amount of resources to be distributed over a wider tangential band of developing cells, rather than only speeding up the processes of differentiation, especially during the resource-demanding wall-thickening phase. It can, therefore, be that elevated auxin concentrations promote the carbon supply required for CW formation (Du and Yamamoto 2007). As such, the biosynthetic pathways providing the substrates for xylogenesis would be coordinated and regulated according to the different demands during wood development via distinct composition, accompanying to different levels of lignin deposition during xylogenesis (Villalobos et al. 2012).
In conclusion, this study, comparing the formation of CW and OW in mountain pine at the treeline on the Majella Massif, provided evidences that in addition to adjusting duration and rates of cell differentiation, the secondary meristem may also modify the width of the differentiating zones, including the enlarging and wall-thickening cells, to achieve very diversified wood structures, despite being produced under the same climatic and site conditions. These evidences of an additional variable involved in regulating wood formation need to be considered when studying the relation between environmental site conditions and wood structure, while deciphering its regulation can be of particular importance for linking environmental conditions to productivity.
Statement on data availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Aloni R, Zimmermann MH (1983) The control of vessel size and density along the plant axis-a new hypothesis. Differentiation 24:203–208
Anfodillo T, Deslauriers A, Menardi R, Tedoldi L, Petit G, Rossi S (2012) Widening of xylem conduits in a conifer tree depends on the longer time of cell expansion downwards along the stem. J Exp Bot 63:837–845
Cailleret M, Jansen S, Robert EMR, Desoto L, Aakala T, Antos JA, Beikircher B, Bigler C, Bugmann H, Caccianiga M, Čada V, Camarero JJ, Cherubini P, Cochard H, Coyea MR, Čufar K, Das AJ, Davi H, Delzon S, Dorman M, Gea-Izquierdo G, Gillner S, Haavik LJ, Hartmann H, Hereş A-M, Hultine KR, Janda P, Kane JM, Kharuk VI, Kitzberger T, Klein T, Kramer K, Lens F, Levanic T, Linares Calderon JC, Lloret F, Lobo-Do-Vale R, Lombardi F, López Rodríguez R, Mäkinen H, Mayr S, Mészáros I, Metsaranta JM, Minunno F, Oberhuber W, Papadopoulos A, Peltoniemi M, Petritan AM, Rohner B, Sangüesa-Barreda G, Sarris D, Smith JM, Stan AB, Sterck F, Stojanović DB, Suarez ML, Svoboda M, Tognetti R, Torres-Ruiz JM, Trotsiuk V, Villalba R, Vodde F, Westwood AR, Wyckoff PH, Zafirov N, Martínez-Vilalta J (2017) A synthesis of radial growth patterns preceding tree mortality. Glob Chang Biol 23:1675–1690
Castagneri D, Fonti P, von Arx G, Carrer M (2017) How does climate influence xylem morphogenesis over the growing season? Insights from long-term intra-ring anatomy in Picea abies. Ann Bot 19:1011–1020. https://doi.org/10.1093/aob/mcw274
Cocozza C, Palombo C, Tognetti R, La Porta N, Anichini M, Giovannelli A, Emiliani G (2016) Monitoring intra-annual dynamics of wood formation with microcores and dendrometers in Picea abies at two different altitudes. Tree Physiol 36:832–846. https://doi.org/10.1093/treephys/tpw009
Côté WA, Day AC, Timell TE (1968) Studies on compression wood VII. Distribution of lignin in normal and compression wood of tamarack Larix laricina (Du Roi, K. Koch). Wood Sci Technol 2:13–37
Cuny HE, Rathgeber CBK, Lebourgeois F, Fortin M, Fournier M (2012) Life strategies in intra-annual dynamics of wood formation: example of three conifer species in a temperate forest in north-East France. Tree Physiol 32:612–625
Cuny HE, Rathgeber CBK, Kiessé TS, Hartmann FP, Barbeito I, Fournier M (2013) Generalized additive models reveal the intrinsic complexity of wood formation dynamics. J Exp Bot 64:1983–1994. https://doi.org/10.1093/jxb/ert057
Cuny HE, Rathgeber CBK, Frank D, Fonti P, Fournier M (2014) Kinetics of tracheid development explain conifer tree-ring structure. New Phytol 203:1231–1241. https://doi.org/10.1111/nph.12871
Deslauriers A, Fonti P, Rossi S, Rathgeber CBK, Gričar J (2017) Ecophysiology and plasticity of wood and phloem formation. In: Amoroso M, Daniels L, Bäker P, Camarero J (eds) Dendroecology. Springer Ecological Series, 231
Donaldson LA (2001) Lignification and lignin topochemistry - an ultrastructural view. Phytochemistry 57:859–873
Donaldson LA, Singh AP, Yoshinaga A, Takabe K (1999) Lignin distribution in mild compression wood of Pinus radiata D. Don. Can J Bot 77:41–50
Donaldson LA, Grace JC, Downes G (2004) Within tree variation in anatomical properties of compression wood in radiata pine. IAWA J 25:253–271
Du S, Yamamoto F (2007) An overview of the biology of reaction wood formation. J Integr Plant Biol 49:131–143
Eilmann B, Zweifel R, Buchmann N, Fonti P, Rigling A (2009) Drought-induced adaptation of the xylem in Scots pine and pubescent oak. Tree Physiol 29:1011–1020
Fonti P, Jansen S (2012) Xylem plasticity in response to climate. New Phytol 195:734–736
Fonti P, Von Arx G, García-González I, Eilmann B, Sass-Klaassen U, Gärtner H, Eckstein D (2010) Studying global change through investigation of the plastic responses of xylem anatomy in tree rings. New Phytol 185:42–53. https://doi.org/10.1111/j.1469-8137.2009.03030.x
Fonti P, Heller O, Cherubini P, Rigling A, Arend M (2013) Wood anatomical responses of oak saplings exposed to air warming and soil drought. Plant Biol 15:210–219
Frankenstein C, Eckstein D, Schmitt U (2005) The onset of cambium activity – a matter of agreement? Dendrochronologia 23:57–62
Galle A, Esper J, Feller U, Ribas-Carbo M, Fonti P (2010) Responses of wood anatomy and carbon isotope composition of Quercus pubescens saplings subjected to two consecutive years of summer drought. Ann For Sci 67:809–809
Gardiner B, Macdonald E (2005) Compression wood in conifers – the characterization of its formation and its relevance to timber quality, in European Union – Framework Programme FP5 – Quality of Life and Management of Living Resources, QLRT-2000-00177, pp 376
Gričar J, Čufar K (2008) Seasonal dynamics of phloem and xylem formation in silver fir and Norway spruce as affected by drought. Russ J Plant Physiol 55:538–543
Gričar J, Zupančič M, Čufar K, Koch G, Schmitt U, Oven P (2006) Effect of local heating and cooling on cambial activity and cell differentiation in the stem of Norway spruce (Picea abies). Ann Bot 97:943–951
Gričar J, Zupančič M, Čufar K, Oven P (2007) Regular cambial activity and xylem and phloem formation in locally heated and cooled stem portions of Norway spruce. Wood Sci Technol 41:463–475
Groover A (2016) Gravitropisms and reaction woods of forest trees – evolution, functions and mechanisms. New Phytol 211:790–802. https://doi.org/10.1111/nph.13968
Heinrich I, Gärtner H (2008) Variations in tension wood of two broad-leaved tree species in response to different mechanical treatments: implications for dendrochronology and mass movement studies. Int J Plant Sci 169:928–936. https://doi.org/10.1086/589695
Körner C (2006) Significance of temperature in plant life. In: Morison JIL, Morecroft MD (eds) Plant growth and climate change. Blackwell Publishing Ltd, Oxford, pp 48–69
Lehner G, Lütz C (2003) Photosynthetic functions of cembran pines and dwarf pines during winter at timberline as regulated by different temperatures, snow cover and light. J Plant Physiol 160:153–166
Lempereur M, Martin-StPaul NK, Damesin C, Joffre R, Ourcival JM, Rocheteau A, Rambal S (2015) Growth duration is a better predictor of stem increment than carbon supply in a Mediterranean oak forest: implications for assessing forest productivity under climate change. New Phytol 207:579–590. https://doi.org/10.1111/nph.13400
Moser L, Fonti P, Buentgen U, Franzen J, Esper J, Luterbacher J, Frank D (2010) Timing and duration of European larch growing season along altitudinal gradients in the Swiss Alps. Tree Physiol 30:225–233
Nanayakkara B, Manley-Harris M, Suckling ID, Donaldson LA (2005) Chemical characterisation of compression wood in Pinus radiata. In: 13th International symposium on wood and pulping chemistry. Auckland, New Zealand
Nanayakkara B, Manley-Harris M, Suckling ID, Donaldson LA (2009) A quantitative chemical indicator of compression wood severity. Holzforschung 63:431–439
Piermattei A, Crivellaro A, Carrer M, Urbinati C (2015) The “blue ring”: anatomy and formation hypothesis of a new tree-ring anomaly in conifers. Trees 29:613–620. https://doi.org/10.1007/s00468-014-1107-x
Prislan P, Schmitt U, Koch G, Gričar J, Čufar K (2011) Seasonal ultrastructural changes in the cambial zone of beech (Fagus sylvatica) grown at two different altitudes. IAWA J 32:443–459
Pya N, Wood SN (2015) Shape constrained additive models. Stat Comput 25:543. https://doi.org/10.1007/s11222-013-9448-7
R Development Core Team (2007) R: a language and environment for statistical computing. R Development Core Team, Vienna
Rathgeber CBK (2012) Cambial activity and wood formation: data manipulation, visualisation and analysis using R. R package version 1.4–1
Rathgeber C, Cuny H, Fonti P (2016) Biological basis of wood formation: a crash course. Front Plant Sci 7:734. https://doi.org/10.3389/fpls.2016.0073
Rossi S, Anfodillo T, Menardi R (2006a) Trephor: a new tool for sampling microcores from tree stems. IAWA J 27:89–97
Rossi S, Deslauriers A, Anfodillo T (2006b) Assessment of cambial activity and xylogenesis by microsampling tree species: an example at the alpine timberline. IAWA J 27:383–394
Rossi S, Deslauriers A, Anfodillo T, Carrer M (2008a) Age-dependent xylogenesis in timberline conifers. New Phytol 177:199–208
Rossi S, Deslauriers A, Gričar J, Seo JW, Rathgeber CBK, Anfodillo T, Morin H, Levanic T, Oven P, Jalkanen R (2008b) Critical temperatures for xylogenesis in conifers of cold climates. Glob Ecol Biogeogr 17:696–707
Rossi S, Morin H, Tremblay MJ (2010) Growth and productivity of black spruce (Picea mariana) belonging to the first cohort in stands within and north of the commercial forest in Quebec, Canada. Ann For Sci 67:807. https://doi.org/10.1051/forest/2010043
Rossi S, Morin H, Deslauriers A (2012) Causes and correlations in cambium phenology: towards an integrated framework of xylogenesis. J Exp Bot 63:2117–2126
Rossi S, Anfodillo T, Čufar K, Cuny HE, Deslauriers A, Fonti P, Frank D, Gričar J, Gruber A, Huang J, Jyske T, Kašpar J, King G, Krause C, Liang E, Mäkinen H, Morin H, Nöjd P, Oberhuber W, Prislan P, Rathgeber CB, Saracino A, Swidrak I, Treml V (2016) Pattern of xylem phenology in conifers of cold ecosystems at the Northern Hemisphere. Glob Chang Biol 22:3804–3813. https://doi.org/10.1111/gcb.13317
Schweingruber FH (2006) Wood structure and environment. Springer-Verlag, Berlin Heidelberg
Singh AP, Donaldson LA (1999) Ultrastructure of tracheid cell walls in radiata pine (Pinus radiata D. Don) mild compression wood. Can J Bot 77:32–40
Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, eds MHL (2007) Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. Climate change 2007: The Physical Science Basis. Cambridge University Press, Cambridge, p 996
Timell TE (1973) Ultrastructure of the dormant and active cambial zones and the dormant phloem associated with formation of normal and compression woods in Picea abies (L.) Karst. Technical publication n.96. State University of New York, College of Environmental Science and Forestry. Siracuse, New York
Timell TE (1986a) The structure of compression wood. In Compression Wood in Gymnosperms, vol 1. Springer-Verlag, Berlin
Timell TE (1986b) Chemical properties of compression wood. In Compression Wood in Gymnosperms, vol 1. Springer-Verlag, Berlin
Villalobos DP, Díaz-Moreno SM, Said E-SS, Cañas RA, Osuna D, Van Kerckhoven SHE, Bautista R, Claros MG, Cánovas FM, Cantón FR (2012) Reprogramming of gene expression during compression wood formation in pine: coordinated modulation of S-adenosylmethionine, lignin and lignan related genes. BMC Plant Biol 12:100
Wood SN (2017) Generalized additive models: an introduction with R, 2nd edn. Chapman and Hall/CRC
Yamamoto H, Okuyama T, Yoshida M, Sugiyama K (1991) Generation process of growth stresses in cell walls. III. Growth stress in compression wood. Mokuzai Gakkaishi 37:94–100
Yamashita S, Yoshida M, Takayama S, Okuyama T (2007) Stem-righting mechanism in gymnosperm trees deduced from limitations in compression wood development. Ann Bot 99:487–493
Yumoto M, Ishida S, Fukazawa K (1983) Studies on the formation and structure of compression wood cells induced by artificial inclination in young trees of Picea glauca. IV. Gradation of the severity of compression wood tracheids. Research Bulletins of the College Experiment Forests Hokkaido University 40: 409
Acknowledgements
We thank the Majella National Park and Ufficio Territoriale per la Biodiversità di Pescara (Carabinieri Forestali) for permission to access the sites and to core the trees. We are grateful to Luciano Schiazza, Giuseppe D’Ascanio, Gianni Blasioli, Roberto Lezzi (Caramanico Terme) for their support in fieldwork, and to Prof. Stefania Scippa and Dr. Dalila Trupiano for providing laboratory equipment, and Dr. Holger Gärtner and Prof. Fritz Schweingruber for valuable suggestions and fruitful discussion.
Funding
This study was financially supported by the Swiss National Science Foundation SNF (projects CLIMWOOD-160077 and LOTFOR-150205).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Cyrille B. K. Rathgeber
Contribution of the co-authors CP carried out the sampling and sample preparation, performed research, analyzed the data, and wrote the paper; PF contributed to sample preparation, analyzed the data, and wrote the paper; BL analyzed the data and wrote the paper; PC wrote on the paper; MM provided new insights; RT provided new insights and wrote the paper.
Electronic supplementary material
ESM 1
(DOCX 26202 kb)
Rights and permissions
About this article
Cite this article
Palombo, C., Fonti, P., Lasserre, B. et al. Xylogenesis of compression and opposite wood in mountain pine at a Mediterranean treeline. Annals of Forest Science 75, 93 (2018). https://doi.org/10.1007/s13595-018-0773-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13595-018-0773-z