Abstract
The aim of this work was to evaluate antimicrobial activity against Paenibacillus larvae and oral toxicity against workers and larvae of Apis mellifera of gallic acid (GA) and two nanohybrids of GA and silica. Also, the physicochemical, structural, and energetic properties of GA and the nanohybrids were determined through structure–activity relationship (SAR). The minimum inhibitory concentration (MIC) against P. larvae was determined. GA showed MIC values between 62.5 and 125 μg/ml, whereas the nanoparticle functionalized through the GA carboxylic moiety (NP2) showed the best antimicrobial activity with a MIC value of 23 μg GA/ml for four of the five isolates used. SAR analysis showed that electronegativity, chemical hardness, and dipolar moment are reliable estimators of the antimicrobial activity. NP2 showed the lowest toxicity against workers and was innocuous for bee larvae. Therefore, the nanohybrid NP2 was the best antibacterial and resulted in non-toxic against workers and larvae of honeybees, becoming a potentially effective and safe agent for the treatment of American Foulbrood disease.
Similar content being viewed by others
1 Introduction
Honeybees Apis mellifera (Hymenoptera: Apidae) are ecologically important as pollinators of a large diversity of plant species (Klein et al. 2007) but are affected by a variety of pest and environmental threats (Genersch 2010). Paenibacillus larvae is the main pathogen of the bee brood, and it is the causative agent of American Foulbrood (AFB) disease (Shimanuki 1997). It is a Gram-positive and spore forming bacillus. Only the spore is able to infect initial larval stages, without being infectious for the adult bee, which acts as an asymptomatic carrier in the disease transmission. Larvae ingest spores that germinate into their midgut, crossing the intestinal wall and causing the larval death (Djukic et al. 2014).
The prevention and treatment of this disease have been usually carried out using synthetic compounds such as oxytetracycline hydrochloride (OTC). This antibiotic has been used for decades in AFB control but has already been banned in Argentina (SENASA 2016). Moreover, its use in bee colonies has caused contamination of honey and beeswax (Bogdanov 2006; Martel et al. 2007) and has led the development of resistant strains of P. larvae (Evans 2003). These negative consequences have prompted the search for new eco-friendly ways to prevent and control this disease. Some volatile and hydroalcoholic botanical extracts and their components have shown in vitro antibacterial activity against P. larvae (Gende et al. 2008; Damiani et al. 2014). Bee venom has been also proposed as an antimicrobial agent of control (Fernández et al. 2014). Meanwhile, cinnamon oil has been successfully proved to control AFB in apiary trials (Gende et al. 2009).
Polyphenols are a ubiquitous group of secondary metabolites produced by plants with important roles in their physiology and ecology, involved as growth regulators, signaling molecules in reproduction, and resistance to pathogens and predators (Bravo 1998). Gallic acid (GA) is a phenolic acid widely distributed in the plant kingdom, abundant in nuts, grapes, cherries, wine (Shukla et al. 1999; Yilmaz and Toledo 2004), and honey (Pyrzynska and Biesaga 2009). As a natural antioxidant, it is able to react with reactive oxygen species (Yilmaz and Toledo 2004; Marino et al. 2014) granting antitumoral activity (Locatelli et al. 2013). On the other hand, antifungal (Chanwitheesuk et al. 2007) and antibacterial activities of GA (Taguri et al. 2006; Borges et al. 2013) have also been demonstrated. The antimicrobial activity of GA has been studied against human pathogens such as Escherichia coli, Pseudomonas aeruginosav, Staphylococcus aureus, and Listeria monocytogenes (Taguri et al. 2006; Chanwitheesuk et al. 2007; Borges et al. 2013). GA acts by causing irreversible changes in the bacterial membrane, altering its hydrophobicity and producing local ruptures (Borges et al. 2013).
GA, as some polyphenols, is susceptible to oxidation in the presence of oxygen. This property reduces their useful life and bioavailability as medication for the organisms (Deligiannakis et al. 2012). The oxidation susceptibility can be reduced through the bonding to different organic or inorganic matrices (Giannakopoulos et al. 2006; Cho et al. 2011). Silica is often used as an inorganic matrix where a bioactive molecule can be bonded to form a hybrid that exhibits characteristics of both compounds (Jin et al. 2009; Deligiannakis et al. 2012). Silica nanoparticles (NPs) have superficial silanol groups (Si–OH) able to act as molecule binding sites (Arce 2010). Furthermore, as they are non-toxic, the NPs find their application in medical and veterinary investigations (Jin et al. 2009). Nanohybrids from GA have shown to be highly stable and have succeeded in preserving antioxidant activity and the reducing oxidation of GA (Panagiota et al. 2009; Hu et al. 2013).
Structure–activity relationship (SAR) studies are very useful tools in investigating the relation between the chemical structure of a compound and its bioactivity (Chang et al. 2007). This kind of analysis is performed by computational calculation of chemical descriptors of physicochemical, constitutional, geometric, topological, or electronic type. The data obtained from these descriptors are then used to explain the biological activity of the molecules studied (Ensuncho et al. 2017).
The emergence of nanoscience and nanotechnology in the last decade presents opportunities for exploring bioactive capacities of nanoparticles. However, few studies have considered the use of nanoparticles as an alternative treatment of honeybee diseases (Vianna Santos et al. 2014; de Almeida Vaucher et al. 2015; Khan et al. 2018). Vico et al. (2016) characterized nanohybrids of GA and silica nanoparticles chemically and determined their antimicrobial activity on a single P. larvae strain. They demonstrated that nanohybrids are more effective against bacteria as compared to free GA, suggesting the use of these materials as good alternative for AFB disease treatment. We here present a more integral work in which both the pathogen and the host are taken into account.
The aim of this work was to study the bioactivity of gallic acid (GA) and gallic acid–loaded silica nanoparticles (GA-NPs) against Paenibacillus larvae and Apis mellifera. In particular, antimicrobial activity against P. larvae and oral toxicity against workers and larvae of honeybees were tested. Moreover, the physicochemical properties of GA and GA-NPs molecules were determined through SAR to explain differences in their bioactivity.
2 Materials and methods
2.1 Chemical material
GA monohydrate (≥ 98% purity, Panreac Chemicals SLU) and NPs (SiO2) (specific surface area of 390 ± 40 m2/g with an estimated particle diameter of 7 nm; Sigma-Aldrich) were used. The NP1 were prepared by condensation of the silanol groups with the phenolic OH of GA (Figure 1a), whereas the NP2 were synthesized from silica NPs previously modified with 3-aminopropyltriethoxysilane and their subsequent union through an amide bond to the carboxyl group of GA (Figure 1b) according to Vico et al. (2016). The percentages of organic groups (w/w) for the functionalized nanoparticles were 17.8% for NP1 and 9.2% for NP2. Also, functionalization of SiO2 by a covalent bond in the meta position of GA was confirmed, although a minor proportion of GA linked to the para position could not be discarded in NP1 (Vico et al. 2016).
2.2 Microorganisms and culture media
A collection of P. larvae isolates from the Center of Research in Social Bees, National University of Mar del Plata was used. Five isolates were maintained on Mueller–Hinton broth, yeast extract, glucose, and sodium pyruvate (MYPGP) agar with 15% (v/v) glycerol until use (Gende et al. 2010). All isolates were biochemically and genotypically identified using PL2-Fw and PL2-Rv primers (Martínez et al. 2010) and characterized with ERIC1R and ERIC2 primers (Versalovic et al. 1994). Two reference strains from OIE Reference Laboratories for AFB acquired in UB-CIDEFI were used.
2.3 Determination of minimal inhibitory concentration by the microdilution method
Vegetative cells of P. larvae were suspended in distilled sterile water (0.5 McFarland scale). The bacterial isolates were exposed to serial dilutions of each individual antimicrobial agent in MYT (Mueller–Hinton broth, yeast extract, and thiamine) aqueous medium (pH7) according to Gende et al. (2010). GA, NPs, NP1, and NP2 aqueous solutions were tested. The concentration range was set between 1.9 and 4000 μg/ml for GA and between 31 and 1333 μg/ml for NPs, NP1 and NP2. Blends were incubated at 35–37 °C ± 0.5 for 48 h. The inhibition of bacterial growth was confirmed by the absence of turbidity. The lowest concentration of the antimicrobial substance that showed inhibition was considered as the minimal inhibitory concentration (MIC) (CLSI 2018). Each treatment was made in triplicate.
2.4 Experimental animals
All experiments were conducted during the spring. Healthy honeybee colonies settled in Langstroth hives from the experimental apiary of the National University of Mar del Plata, Argentina (38° 10′ 06″ S, 57° 38′ 10″ W) were used. The colonies were controlled in order to maintain a low abundance of Nosema ceranae and a low prevalence of Varroa destructor mites; neither showed any visible clinical symptoms of other diseases (i.e., AFB, Chalkbrood, or viruses). To worker bee toxicity tests, capped brood combs were got from three colonies and kept under laboratory conditions at 32 ± 1 °C and 60% RH until the imagoes emerged. Brood combs with 1-day-old bee larvae were used for in vitro toxicity test against larvae.
2.5 Oral toxicity to adult honeybees
Oral toxicity against workers of different concentrations of GA, NPs, and GA-NPs added to the food was tested. Twenty-five newly emerged bees were confined in each 15 × 15 × 3-cm wooden box with one metal mesh side wall and the other made of transparent acrylic. Each unit was provided with one device with water and another containing the food. Substance doses were incorporated into a powdered sugar–based food (candy) and supplied ad libitum to bees. The concentrations tested were 14, 50, 70, 100, and 140 mg/g of food for GA, NP1, and NP2 and 2, 4, 8, 12, and 18 mg/g of food for NPs. NPs were tested at lower concentration due to the low density of these nanoparticles, which prevented preparing the food at higher concentrations. Nurse bees were maintained under short-term consumption of the different substances during nine successive days. The control was carried out by feeding bees only with sugar candy. Each treatment and the control were tested in triplicate. The food was supplied daily, and consumption was recorded simultaneously. Using a removable device, the food mass was measured and the amount consumed was divided by the number of live bees.
2.6 Oral toxicity to honeybee larvae
Taking into account the results obtained after evaluating in vitro antimicrobial activity and oral toxicity against worker bees, we decided to test the toxicity against larvae only for NP2 nanohybrid, since it showed the lowest MIC and was not toxic for bees. Larvae were fed according to Aupinel et al. (2005) protocol. The diets were prepared by adding different concentrations of NP2 nanohybrid (1, 2.5, 5, and 10 mg/ml) and maintained until the last larval state. The highest concentration was estimated according to the maximum saturation of NP2 in aqueous solution. As a control group, larvae were maintained with regular feeding all the test. Twenty-four larvae per treatment and control were used. Devices were incubated at 34 ± 0.5 °C and 90% RH. Larval mortality was daily registered under a dissecting microscope.
2.7 Statistical analysis
Survival curves (number of live bees versus time for each treatment) and LT50 values (median lethal time after exposure of an organism to a toxic substance or stressful condition) were performed by the Kaplan–Meier method (Kaplan and Meier 1958). The non-parametric log-rank test was performed to determine differences between survival curves. Multiple comparisons between groups were made by Holm–Sidak and Bonferroni methods. Worker bee preference for diet was assessed by comparing the daily mean feed intake by means repeated measure ANOVA test. Fisher LSD (least significant difference) post hoc tests (α = 0.05) were performed to compare among groups. LD50 values (amount of a substance that is sufficient to kill 50% of a population of animals exposed within a certain time) for workers and larvae were estimated on the 9th and 6th days of life, respectively. Selectivity ratios were calculated by means the formula of Lindberg et al. (2000) adapted as LD50 for workers or larvae/MIC average for P. larvae for each molecule tested. LD50 values and MIC were expressed in parts per million to unify concentration units. This index can be used as an estimate of the safety factor for applying a substance in field formulations.
2.8 Structure–activity relationship
Computational chemistry was used to study the chemical structure of GA and their nanohybrids. In order to use short periods of computing, a silica base particle was built (SiBP). The SiBP showed the following molecular formula, Si5O13H2, and it was used in theoretical quantum calculations displayed in this work. From thermogravimetric data of the NP1 and NP2 particles (Vico et al. 2016), we calculated the amount of SiBP in them (2 for NP1 and 4 for NP2). Geometric optimizations for all three were performed. The molecular reactivity depends on the electronic structure, and it can be estimated by using molecular descriptors (molecular diameter, molecular volume, dipole moment (μ), maps of electrostatic potential (MEP), energetic data of LUMO (lowest unoccupied molecular orbital) (EL) and HOMO (highest occupied molecular orbital) (EH)). These descriptors were calculated with the density functional theory implemented by software Spartan 8 which allows calculating mathematical expressions for quantifying molecular reactivity and to simulate the electrostatic potential maps. Other descriptors such as electronegativity (En), chemical hardness (η), and electrophilicity index (ω) (Pearson 1986; Geerlings et al. 2003) were also taken into account. En and η were initially expressed as a function of the ionization potential and the electron affinity, but later, they were proposed in terms of the energies of the HOMO and LUMO, EH and EL, considering the validity of the Koopmans’ theorem Eqs. (1) and (2) (see below). Koopmans’ theorem neglects the change in the form of the molecular orbital that occurs when the molecule is ionized (Hinde 2000). The units of all the indexes, except for the dipole moment, are in electron volts (eV). It is important to note that the last descriptors are all function of EH and EL indexes, calculated by the software used. The energy gap between the HOMO occupied orbital by electrons and the LUMO unoccupied orbital has particular importance. Equations (1)–(3) show how En, η, and ω were calculated.
3 Results
3.1 Antimicrobial activity against Paenibacillus larvae
The five P. larvae isolates were characterized as ERIC I. The results of antimicrobial activity of the different substances against five isolates are shown in Table I. MIC values were reported according to Clinical and Laboratory Standards Institute recommendations (CLSI 2012). For the case of NP1 and NP2, the results are relativized to the percentages of organic functional groups bound to the silica nanoparticles.
GA showed bacterial growth inhibition values between 62.5 and 125 μg/ml, whereas NPs demonstrated no antimicrobial activity in the range of concentrations tested. NP2 showed the best inhibitory activity with a MIC value of 23 μg GA/ml for four of the five isolates studied and a value greater than 122 μg GA/ml for the isolate from Cobo.
3.2 Oral toxicity against worker bees and food consumption
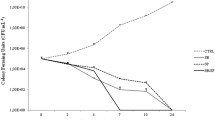
GA, NP1, and NP2 showed differential toxicity against adult bees. In all cases, mortality was directly proportional to the concentration of the substance in the food, except for the unmodified silica nanoparticles that resulted to be no toxic at all concentrations tested (χ2 = 6.875; df = 5; p = 0.230). For GA, all survival curves were significantly different from the control and to each other (χ2 = 447.24; df = 5; p < 0.001) (Figure 2). For NP1 and NP2 nanoparticles, no significant differences between the control and the lowest concentration (14 mg/g) survival curves were found (χ2 = 1.39; p = 0.237 and χ2 = 0.96; p = 0.546, respectively), although NP1 turned out to be more toxic than NP2 at higher concentrations (Figure 2). From both nanohybrids, NP1 nanoparticle was the most toxic showing LT50 values and 95% confidence interval of 7 (5.12–8.87), 5 (4.60–5.40), 4 (3.30–4.69), and 3 (2.7–3.29) days old at 50, 70, 100, and 140 mg/g food, respectively, whereas LT50 values for NP2 were higher than 9 days at 50, 70, and 100 mg/g food and than 8 (7.15–8.84) days at 140 mg/g food. The LT50 values for GA were 4 (3.62–4.37), 4 (3.78–4.21), 3 (2.84–3.15), and 3 (2.81–3.18) days at 50, 70, 100, and 140 mg/g food, respectively.
Bee survival curves after the consumption of food with GA, NPs, NP1, and NP2 by the Kaplan–Meier method/log-rank test. GA gallic acid, NPs silica nanoparticles; NP1: silica nanoparticles bound to gallic acid by alkoxide type bonding; NP2: silica nanoparticles functionalized with gallic acid by amide bonding. N = 75 bees per treatment and concentration. Concentrations are expressed in milligrams per gram of food. Different letters indicate significant differences (p < 0.05).
Figure 3 shows the results of food consumption during the 9 successive days of treatment. Only the feed with GA incorporated showed significant differences in the consumption per bee depending on the concentration (F = 23.86; df = 5; p = 0.000029), where those of higher concentration ones were the less consumed. For NPs, NP1, and NP2 groups, the bees consumed the food regardless of the concentration (F = 0.87; df = 4; p = 0.52 for NPs, F = 1.65; df = 5; p = 0.22 for NP1, and F = 1.19; df = 5; p = 0.36 for NP2). In any case, significant interactions between the concentration and the treatment time were found for GA, NP1, and NP2 (F = 2.88; df = 40; p = 0.000028 for GA, F = 3.23; df = 40; p = 0.000001 for NP1, and F = 2.29; df = 40; p = 0.00051 for NP2).
Average consumption (± SE) of food per bee (mg/bee). GA gallic acid, NPs silica nanoparticles; NP1: silica nanoparticles bound to gallic acid by alkoxide type bonding; NP2: silica nanoparticles functionalized with gallic acid by amide bonding. N = 75 bees per treatment and concentration. Concentrations are expressed in milligrams per gram of food. The different letters indicate significant differences (p < 0.05) between different days for a single concentration. Single and double asterisks indicate significant differences (p < 0.05) between concentrations within the same day.
3.3 Oral toxicity against larvae honeybee
Survival analysis showed significant differences among survival curves of honeybee larvae fed with different concentrations of NP2 (χ2 = 9.75; df = 4; p = 0.045) (Figure 4). However, multiple comparisons between groups did not find differences (All p values greater than 0.05). LT50 values could not be determined for this survival analysis.
3.4 Selectivity index
LD50 values and selectivity ratios were calculated for the substance tested against worker and larva bees (Table II). NPs were no toxic for workers and their LD50 values were > 18,000 ppm. Compared to worker bees, larvae were more susceptible to NP2 as LD50 values of 36,550 ppm and selectivity ratios of 1589 were obtained. In addition, this nanohybrid showed a better selectivity index for workers (3982) than NP1 and GA, with 683.1–170.8 and 155.2–77.5, respectively.
3.5 Structure–activity relationship
Table III shows the values of molecular descriptors calculated computationally under the theory of density functional and the values of electronegativity, hardness, and electrophilicity calculated by Eqs. 1, 2, and 3, respectively. The highest values of hardness and electronegativity were obtained for SiBP. The nanoparticle NP2 showed the lowest values of electrophilicity and electronegativity.
The MEP used to investigate the molecular interactions paints the value of the electrostatic potential onto an electron density surface. Red colors represent negative potential or an electron rich surface; colors toward blue depict positive potential or electron poor surface, whereas orange, yellow, and green colors depict intermediate values of the potential. The red–orange–yellow–green–blue color scale only provides information about the contact surface, and it does not reveal to what extent the rich and electron poor areas extend beyond the surface. These maps allow us to understand that the molecules prefer to interact joining their regions of high and low electron density that is red regions of a molecule with blue ones of the other. The interaction is based on the electrical forces and will be more intense as the red and blue colors become stronger. The purpose of the map is to display an easy visualization where a numeric scale could not fulfill that role. The maps of the molecules investigated are shown in Figure 5. NP1 and NP2 showed zones with high electron density in close proximity to the carboxyl group and the phenoxide group, respectively. NP2 also displayed two other high-density zones, one of them in the phenolic groups of the GA and the other in the amide group. The theoretical acidity constant (pKa) for GA was –COOH = 4.5 and –OH = 10.0, whereas the pKa for SiBP was –OH = 9.84.
Chemical structures optimized by the density functional method and electrostatic potential maps of GA, SiBP, NP1, and NP2. GA gallic acid, SiBP silica base particles; NP1: silica nanoparticles bound to gallic acid by ether type bonding; NP2: silica nanoparticles functionalized with gallic acid by amide bonding.
4 Discussion and conclusion
Phenolic compounds of plant origin have been shown to be active against a wide range of pathogenic bacteria (Taguri et al. 2006; Sánchez-Maldonado et al. 2011). The antimicrobial capacity of these compounds has been related to their chemical structure, in particular to the number and position of hydroxy and methoxy substituents (Sánchez-Maldonado et al. 2011). Substances with a benzene nucleus, such as GA, commonly exhibit good antimicrobial activity attributed to a greater offshoring in the electronic system, which allows proton exchange by making them more active against microorganisms (Ben Arfa et al. 2006).
In the present work, MIC values for GA against five isolates of P. larvae ranged from 62.5 to 125 μg/ml. Our results showed differences with those obtained by Vico et al. (2016) who obtained an inhibition concentration of 2500 μg GA/ml by the dilution method on a solid medium (MYPGP agar). This discrepancy can be attributed to different techniques for MIC determination used in each work. In the presence of oxygen and at neutral pH, GA is oxidized and produces significant amounts of hydrogen peroxide (H2O2) and free radical semiquinone (Akagawa et al. 2003; Eslami et al. 2010). While the anaerobic condition of a solid medium completely inhibits the generation of H2O2, the MYT aqueous medium used here would have promoted the production of free radicals that explain the greater antimicrobial activity recorded (Arakawa et al. 2004; Nakamura et al. 2012). Nakamura et al. (2012) proposed a mechanism of bactericidal action for GA. Through the photo-oxidation, GA transfers a proton-coupled electron to the oxygen to form H2O2 which by photolysis releases the free radical (·OH) causing lethal oxidative damage including lipid peroxidation of the bacterial cells. Therefore, to determine the capacity of inhibition of bacterial growth, the properties of the natural substances must be taken into account, in order to choose the technique that best demonstrates their bioactivity. In our case, the antimicrobial activity was depended on the solubility of the substances in an aqueous medium, feature determined by their hydrophobicity, volatility, and stability (Ahmad et al. 2006).
The microdilution technique has given satisfactory results by testing a wide range of natural substances against P. larvae (Damiani et al. 2014; Fernández et al. 2014; Gende et al. 2008, 2010). Moreover, it has also been used to study the antimicrobial activity of silica, silver, gold, zinc, and copper nanoparticles against a large number of bacterial strains (Martínez-Castañon et al. 2008; Moreno-Álvarez et al. 2010; Duffy et al. 2018). For that, the reproducibility and reliability in our results are guaranteed.
Currently, to develop controlled drug delivery systems that improve the pharmacokinetic and pharmacodynamic properties of substances is of great interest to the field of medicine and veterinary (Mohanraj and Chen 2006; Yah and Simate 2015). Nanohybrids with different characteristics have been studied and demonstrated to improve significantly the bioactivities of many natural substances compared with their free state (Yang et al. 2009; Vianna Santos et al. 2014; de Almeida Vaucher et al. 2015). In the present work, the nanohybrids NP1 and NP2 synthesized from the binding of GA molecules and silica nanoparticles (NPs) were tested. The nanohybrid NP1 presents an ether-like bond between GA and NPs, whereas NP2 has an amide-like bond. Both nanohybrids were studied here to determine their potential as antimicrobial agents against various isolates of P. larvae. The MIC values on P. larvae were 44–178 μg GA/ml and 23 μg GA/ml for NP1 and NP2, respectively, except for the isolate from Cobo which showed a value > 122 μg GA/ml for NP2. The bioactivity of the nanohybrid NP1 was comparable to that of the free GA in the presence of oxygen and at neutral pH. Thus, our results suggest that this type of surface chemical modification does not improve the activity of GA in its free state. On the other hand, the MIC values for NP2 were 4-fold lower than the GA ones for P. larvae isolates from Estafeta and Miramar and the reference strains PL33 and PL15. Vico et al. (2016) reported an inhibition concentration on P. larvae of 178 μg GA/ml for NP1 and 92 μg GA/ml for NP2. These authors suggested that when GA is covalently bound to silica nanoparticles, it increases its bioactivity on the microorganisms.
This increment relates to free functional molecules and was also reported by Arce et al. (2012) in a toxicity study of silica nanoparticles modified with alcohols against Vibrio fischeri. They hypothesized that suspended nanoparticles form aggregates which increase their local concentration on the surface of the microorganism, facilitating the bactericidal action of these materials. Hetrick et al. (2008) concluded similarly about the possible mechanism of action of nanohybrids of nitric oxide (NO) and silica. They proposed an association mechanism between the bacterial membrane and nanohybrids due to electrostatic and/or hydrophobic interactions resulting in a high local concentration of NO and in a greater efficiency of distribution and antimicrobial activity of NO on the bacterial surface. A study with electron paramagnetic resonance (EPR) of the nanohybrids did not show signs of production of reactive oxygen species at pH 7 (Vico et al. 2016); therefore, a similar mechanism of action to GA cannot be attributed to these compounds.
The study of the chemical structure and electronic characteristics of molecules helps to understand and explain the differences in their bioactivity. In this work, computational simulations were carried out and the optimized structures and electronic descriptors were obtained for all molecules under study. The chemical reactivity of a molecule can be explained by means of these descriptors (Carrión 2013). The chemical hardness (η) is a property that measures the resistance imposed by the molecule to the change in its electronic distribution; therefore, higher values of this parameter implicate that the molecule is more stable and less reactive. The results of SAR analysis indicate that SiBP are the least reactive molecules since they showed the highest En and ɳ values. In contrast, NP2 proved to be the most reactive species since its ω and En values were the lowest found.
A MEP is used to study the molecular interactions (Al-Sehemi et al. 2016). In our work, these maps showed that the areas with the highest negative charge in NP1 are located in the carboxylic bond, whereas two negative charge areas, one in the amide bond and another around the hydroxyl group of GA, are found in NP2. The differential antimicrobial activity registered here could be related to these zones, since highly electronegative compounds can inhibit the growth of microorganisms, intervening in the biological processes of electron transfer, either by direct interference of this chain or by rupture of the cell membrane and elimination of the membrane potential (Kurita et al. 1981). Our computational results were in agreement with the experimental results of antimicrobial activity. Therefore, physical characteristics (surface area, aggregate formation) and chemical (type of bonds and energy) could be acting in combination to improve the bioactivity of these nanohybrids against P. larvae.
During the search of substances for the control of honeybee pathogens, the toxicity against the host organism must be evaluated (Eguaras and Ruffinengo 2006). In several studies, the oral toxicity of natural substances such as propolis, organic acids, essential oils, and their main components has been tested against bees (Ebert 2007; Maistrello et al. 2008; Maggi et al. 2010; Damiani et al. 2017; Porrini et al. 2017). Here, the oral toxicity of GA, NP1, NP2, and NPs against workers and toxicity of NP2 against larvae of A. mellifera were proved for the first time. GA turned out the most toxic compound followed by NP1 and NP2, successively. In all cases, the toxicity depended mainly on the concentration administered with the food. The systemic effect on worker bees of NP1 and NP2 appears to be associated with the relative amount of organic compound bound to the silica (17.8 and 9.2% of GA in NP1 and NP2, respectively). Therefore, the GA concentration was directly related to the increases in bee toxicity. GA showed LT50 values similar to amygdalin and oxalic acid, but we tested here a 70 times higher concentration of GA than that used by Ebert et al. (2007). NP1 and NP2 were less toxic than GA and showed LT50 values lower than 9, 5, and 3 days for NP1 and smaller than 9, 9, and 8 days for NP2, at concentrations of 14, 70, and 140 mg/g, respectively. NPs are inert and were not toxic to bees when they were ingested with the sugar food. Regarding the eating, bees ate avidly the supplemented food with NP1, NP2, and NPs independently of the concentration of substance added. However, the food supplemented with GA was less consumed by bees as the concentration increased. Therefore, the bee mortality observed in this group could also be due to the fact that bees were not feeding adequately. Anyway, all the compounds studied here resulted less toxic compared to other substances frequently used in beekeeping such as oxalic acid (Ebert et al. 2007).
Drug administration in bee food has shown to be an efficient form of application of treatments against AFB. Worker bees act as drug deliveries to the larval food, which facilitates the interaction and direct contact of the antimicrobial agent with P. larvae vegetative cells (Antunez et al. 2008). These authors proposed that the oral administration of an effective drug does not prevent the infection of larvae with spores of P. larvae, but it could inhibit the development of vegetative cells in larvae. Due to nurse bee dynamics during brood feeding, an antimicrobial agent would be available in the larval food on the third day of development stage, coinciding with the process of spore germination in the larval midgut.
Based on the previous results, toxicity against larvae was only tested for NP2 nanohybrid. The rest of the substances resulted toxic for workers fed with them. Even exceeding its maximum solubility in aqueous medium, feeding with NP2 was not lethal for bee larvae. As expected, larvae were more susceptible to NP2 than workers although selectivity ratios were high for both stages of life, 3982 and 1589 respectively. The selectivity ratio is a measure to evaluate the safety in the application of a substance in field conditions taking into account the toxicity against the host organism and the desired biological activity, in our case, the antimicrobial activity. Therefore, high values of this index indicate that a substance is safe to apply in honeybee colonies as an AFB treatment. In our case, a concentration 14 mg/g (14,000 ppm) of NP2 in food (the lowest concentration at which the worker bees were fed) was not toxic for bees and it would be sufficient to achieve antimicrobial action as the MIC value for this nanohybrid was 23 μg/ml (23 ppm). The dynamics of a substance inside the hive makes necessary applying doses higher than the MIC value to achieve the desired effect. However, field trials should be performed to know the effective dose of substance to apply in natural conditions. As a reference, in the work of Gende et al. (2009), cinnamon (Cinnamomum zeylanicum) essential oil showed a MIC of 50 ppm on P. larvae, whereas applications of 1000 ppm were needed to reduce the level of AFB in hives. Therefore, it would be expected that doses of around 460 ppm of NP2 are effective under field conditions, much lower values than the LD50 ones obtained for worker bees (96,000 ppm) and larvae (36,000 ppm).
This research represents the first record of oral administration of silica nanoparticles and different GA nanohybrids resulting practically innocuous for bees. Also, it is one of the few investigations of the use of this type of particles for the control of honeybee diseases. We conclude that the toxicity and mode of action of each compound depended on their physical and chemical characteristics, the mode of application, and the organism on which it was applied. Thereby, silica nanoparticles with GA loaded through an amide bond to the carboxyl group, which denominated NP2, presented the best attributes to be used in the control of AFB disease such as the high inhibitory capacity on P. larvae and low oral toxicity against larvae and adults of A. mellifera. Future researches on this kind of substance should be focused on conducting larval infection test and evaluating the behavior of nanohybrids in natural systems such as honeybee colonies.
References
Ahmad, I., Farrukh, A., Mohammad, O. (2006) Modern Phytomedicine, Turning Medicinal Plants Into Drugs. John Wiley & Sons, Weinheim
Akagawa, M., Shigemitsu, T., Suyama, K. (2003) Production of hydrogen peroxide by polyphenols and polyphenol-rich beverages under quasi-physiological conditions. Biosci. Biotechnol. Biochem. 67 (12), 2632–2640
Al-Sehemi, A.G., Irfan, A., Alrumman, S.A., Hesham A.E. (2016) Antibacterial activities, DFT and QSAR studies of quinazolinone compounds. Bull. Chem. Soc. Ethiop. 30 (2), 307–316
Antunez, K., Harriet, J., Gende, L.B., Maggi, M., Eguaras, M., Zunino, P. (2008) Efficacy of natural propolis extract in the control of American Foulbrood. Vet. Microbiol. 131 (3–4), 324–331
Arakawa, H., Maeda, M., Okubo, S., Shimamura, T. (2004) Role of hydrogen peroxide in bactericidal action of catechin. Biol. Pharm. Bull. 27 (3), 277–281
Arce, V.B. (2010) Modificación y caracterización de nanopartículas de sílice. Doctoral thesis. Facultad de Ciencias Exactas, Universidad Nacional de la Plata, Argentina
Arce, V.B., Bertolotti, S.G., Oliveira, F.J., Airoldi, C., Arques, A., Santos-Juanes, L., Gonzalez, MC., Cobos, C.J., Allegretti, P.E., Mártire, D.O. (2012) Triplet state of 4-methoxybenzyl alcohol chemisorbed on silica nanoparticles. Photochem. Photobiol. Sci. 11 (6), 1032–1040
Aupinel, P., Fortini, D., Dufour, H., Tasei, J.N., Michaud, B., Odoux, J.F., Delegue, M.H.P. (2005) Improvement of artificial feeding in a standard in vitro method for rearing Apis mellifera larvae. B. Insectol. 58 (2), 107–111
Ben Arfa, A., Combes, S., Preziosi-Belloy, L., Gontard, N., Chalier, P. (2006) Antimicrobial activity of carvacrol related to its chemical structure. Lett. Appl. Microbiol. 43 (2), 149–154
Bogdanov, S. (2006) Contaminants of bee products. Apidologie 37 (1), 1–18.
Borges, A., Ferreira, C., Saavedra, M.J., Simões, M. (2013) Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 19 (4), 256–265.
Bravo, L. (1998) Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 56 (11), 317–333
Carrión, S.M. (2013) Estudio Teórico de las Propiedades Estructurales, Electrónicas, y de la Reactividad. Doctoral thesis. Facultad de Ciencias Exactas– Universidad Nacional de La Plata. La Plata.
Chang, H.J., Kim, H.J., Chun, H.S. (2007) Quantitative structure–activity relationship (QSAR) for neuroprotective activity of terpenoids. Life Sci. 80 (9), 835–841.
Chanwitheesuk, A., Teerawutgulrag, A., Kilburn, J.D., Rakariyatham, N. (2007) Antimicrobial gallic acid from Caesalpinia mimosoides Lamk. Food Chem. 100 (3), 1044–1048
Cho, Y.S., Kim, S.K., Ahn, C.B., Je, J.Y. (2011) Preparation, characterization, and antioxidant properties of gallic acid-grafted-chitosans. Carbohydr. Polym. 83 (4), 1617–1622
CLSI. (2018) Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically. 11th ed. CLSI standard M07. Wayne, PA: Clinical and Laboratory Standards Institute
Damiani, N., Fernández, N.J., Porrini, M.P., Gende, L.B., Álvarez, E., Buffa, F., Brasesco, C., Maggi, M.D., Marcangeli, J.A., Eguaras, M.J. (2014) Laurel leaf extracts for honeybee pest and disease management: antimicrobial, microsporicidal, and acaricidal activity. Parasitol. Res. 113 (2), 701–709
Damiani, N., Porrini, M.P., Lancia, J.P., Álvarez, E., Garrido, P.M., Domínguez, E., Gende, L.B., Eguaras, M.J. (2017) Effect of propolis oral intake on physiological condition of young worker honey bees, Apis mellifera L. J. Apicult. Sci. 61 (2), 193–202
de Almeida Vaucher, R., Giongo, J.L., Bolzan, L.P., Côrrea, M.S., Fausto, V.P., et al. (2015) Antimicrobial activity of nanostructured Amazonian oils against Paenibacillus species and their toxicity on larvae and adult worker bees. J. Asia Pac. Entomol. 18 (2), 205–210
Deligiannakis, Y., Sotiriou, G.A., Pratsinis, S.E. (2012) Antioxidant and antiradical SiO2 nanoparticles covalently functionalized with gallic acid. ACS Appl. Mater. Interfaces 4 (12), 6609–6617
Djukic, M., Brzuszkiewicz, E., Fünfhaus, A., Voss, J., Gollnow, K., Poppinga, L., Liesegang, H., Garcia-Gonzalez, E., Genersch, E., Daniel, R. (2014) How to kill the honey bee larva: Genomic potential and virulence mechanisms of Paenibacillus larvae. PLoS ONE 9 (3), e90914
Duffy, L.L., Osmond-McLeod, M.J., Judy, J., King, T. (2018) Investigation into the antibacterial activity of silver, zinc oxide and copper oxide nanoparticles against poultry-relevant isolates of Salmonella and Campylobacter. Food Control 92, 293–300.
Ebert, T., Kevan, P., Bishop, B., Kevan, S., Downer, R., (2007) Oral toxicity of essential oils and organic acids fed to honey bees (Apis mellifera). J. Apicult. Res. Bee World 46 (4), 220–224
Eguaras, M., Ruffinengo, S. (2006) Estrategia para el control de Varroa. Ed. Martin, Mar del Plata
Ensuncho, A.E., Robles, J.R., Figueredo, S.F. (2017) Modelado de las relaciones cuantitativas estructura-actividad (QSAR) de los derivados 5-(Nitroheteroaril)-1,3,4-tiadiazol con actividad leishmanicida. Inf. Tecnol. 28 (2), 191–202
Eslami, A., Pasanphan, W., Wagner, B., Buettner, G., (2010) Free radicals produced by the oxidation of gallic acid: An electron paramagnetic resonance study. Chem. Cent. J. 4 (1), 15
Evans, J.D. (2003) Diverse origins of tetracycline resistance in the honey bee bacterial pathogen Paenibacillus larvae. J. Invertebr. Pathol. 83 (1), 46–50
Fernández, N.J., Porrini, M.P., Podaza, E.A., Damiani, N., Liesel, B.G., Eguaras, M.J. (2014) A scientific note on the first report of honeybee venom inhibiting Paenibacillus larvae growth. Apidologie 45 (6), 719–721
Geerlings, P., De Proft, F., Langenaeker, W. (2003) Conceptual density functional theory. Chem. Rev. 103 (5), 1793–1874
Gende, L.B., Floris, I., Fritz, R., Eguaras, M.J. (2008) Antimicrobial activity of cinnamon (Cinnamomum zeylanicum) essential oil and its main components against Paenibacillus larvae from Argentine. B. Insectol. 61 (1), 1–4
Gende, L.B., Maggi, M., Damiani, N., Fritz, R., Eguaras, M.J., Floris, I. (2009) Advances in the apiary control of the honeybee American Foulbrood with Cinnamon (Cinnamomum zeylanicum) essential oil. B. insectol. 62 (1), 93–97
Gende, L.B., Maggi, M., Van Baren, C., Di Leo, A., Bandoni, A., Fritz, R., Eguaras, M.J. (2010) Antimicrobial and miticide activities of Eucalyptus globulus essential oils obtained from different Argentine regions. Span. J. Agric. Res. 8 (3), 642–650
Genersch, E. (2010) Honey bee pathology: current threats to honey bees and beekeeping. Appl. Microbiol. Biotechnol. 87 (1), 87–97
Giannakopoulos, E., Stathi, P., Dimos, K., Gournis, D., Sanakis, Y., Deligiannakis, Y. (2006) Adsorption and radical stabilization of humic-acid analogues and Pb 2+ on restricted phyllomorphous clay. Langmuir. 22 (16), 6863–6873
Hetrick, E.M., Shin, J.H., Stasko, N.A., Johnson, C.B., Wespe, D.A., Holmuhamedov, E., Schoenfisch, M.H. (2008) Bactericidal efficacy of nitric oxide-releasing silica nanoparticles. ACS Nano 2 (2), 235–246
Hinde, R.J. (2000) Quantum Chemistry, Fifth Edition (by I.N. Levine). J. Chem. Educ. 77 (12), 1564
Hu, H., Nie, L., Feng, S., Suo, J. (2013) Preparation, characterization and in vitro release study of gallic acid loaded silica nanoparticles for controlled release. Pharmazie. 68 (6), 401–405
Jin, Y., Li, A., Hazelton, S.G., Liang, S., John, C.L., Selid, P.D., Pierce, D.T., Zhao, J.X. (2009) Amorphous silica nanohybrids: Synthesis, properties and applications. Coord. Chem. Rev. 253 (23–24), 2998–3014
Kaplan, E.L., Meier, P. (1958) Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 53 (282), 457–481
Khan, S.U., Anjum, S.I., Ansari, M.J., Khan, M.H.U., Kamal, S., et al. (2018) Antimicrobial potentials of medicinal plant’s extract and their derived silver nanoparticles: A focus on honey bee pathogen. Saudi J. Biol. Sci., doi: https://doi.org/10.1016/j.sjbs.2018.02.01
Klein, A.M., Vaissiere, B.E., Cane, J.H., Steffan-Dewenter, I., Cunningham, S.A., Kremen, C., Tscharntke, T. (2007) Importance of pollinators in changing landscapes for world crops. Proc. Biol. Sci. 274 (1608), 303–313
Kurita, N., Miyaji, M., Kurane, R., Takahara, Y. (1981) Antifungal activity of components of essential oils. Agric. Biol. Chem. 45 (4), 945–952
Lindberg, C.M., Melathopoulos, A.P., Winston, M.L. (2000) Laboratory evaluation of miticides to control Varroa jacobsoni (Acari: Varroidae), a honey bee (Hymenoptera: Apidae) parasite. J. Econ. Entomol. 93 (2), 189–198
Locatelli, C., Filippin-Monteiro, F.B., Creczynski-Pasa T.B. (2013) Alkyl esters of gallic acid as anticancer agents: A review. Eur. J. Med. Chem. 60, 233–239
Maggi, M.D., Ruffinengo, S.R., Gende, L.B., Sarlo, E.G., Eguaras, M.J, Bailac, P.N., Ponzi, M.I. (2010) Laboratory evaluations of Syzygium aromaticum (L.) Merr. et Perry essential oil against Varroa destructor. J. Essen. Oil Res. 22 (2), 119–122
Maistrello, L., Lodesani, M., Costa, C., Leonardi, F., Marani, G., Caldon, M., Mutinelli, F., Granato, A. (2008) Screening of natural compounds for the control of nosema disease in honeybees (Apis mellifera). Apidologie 39 (4), 436–445
Marino, T., Galano, A., Russo, N. (2014) Radical scavenging ability of gallic acid toward OH and OOH radicals. Reaction mechanism and rate constants from the density functional theory. J. Phys. Chem. B. 118 (35), 10380–10389
Martel, A.C., Zeggane, S., Aurières, C., Drajnudel, P., Faucon, J.P., Aubert, M. (2007) Acaricide residues in honey and wax after treatment of honey bee colonies with Apivar® or Asuntol® 50. Apidologie 38 (6), 534–544
Martínez, J., Simon, V., Gonzalez, B., Conget, P. (2010) A real-time PCR-based strategy for the detection of Paenibacillus larvae vegetative cells and spores to improve the diagnosis and the screening of American foulbrood. Lett. Appl. Microbiol. 50 (6), 603–610
Martínez-Castañon, G.A., Niño-Martínez, N., Martínez-Gutiérrez, F., Martínez-Mendoza, J.R., Ruíz, F. (2008) Synthesis and antibacterial activity of silver nanoparticles with different sizes. J. Nanoparticle Res. 10 (8), 1343–1348
Mohanraj, V.J., Chen, Y. (2006) Nanoparticles - A review. Trop. J. Pharm. Res. 5 (1), 561–573
Moreno-Álvarez, S.A., Martínez-Castañón, G.A., Niño-Martínez, N., Reyes-Macías, J. F., Patiño-Marín, N., Loyola-Rodríguez, J.P., Ruiz, F. (2010) Preparation and bactericide activity of gallic acid stabilized gold nanoparticles. J. Nanoparticle Res. 12 (8), 2741–2746
Nakamura, K., Yamada, Y., Ikai, H., Kanno, T., Sasaki K., Niwano, Y. (2012) Bactericidal action of photoirradiated gallic acid via reactive oxygen species formation. J. Agric. Food Chem. 60 (40), 10048–10054
Panagiota, S., Louloudi, M., Deligiannakis, Y. (2009) EPR study of phenolic radical stabilization by grafting on SiO2. Chem. Phys. Lett. 472 (1–3), 85–89
Pearson, R.G. (1986) Absolute electronegativity and hardness correlated with molecular orbital theory. Proc. Natl. Acad. Sci. U.S.A. 83 (22), 8440–8441
Porrini, M.P., Garrido, P.M., Gende, L.B., Rossini, C., Hermida, L., Marcángeli, J.A., Eguaras, M.J. (2017) Oral administration of essential oils and main components: Study on honey bee survival and Nosema ceranae development. J. Apicult. Res. 56 (5), 616–624
Pyrzynska, K., Biesaga, M. (2009) Analysis of phenolic acids and flavonoids in honey. Trends Analyt. Chem. 28 (7), 893–902
Sánchez-Maldonado, A.F., Schieber, A., Gänzle, M.G. (2011) Structure-function relationships of the antibacterial activity of phenolic acids and their metabolism by lactic acid bacteria: Antibacterial phenolic acids. J. Appl. Microbiol. 111 (5), 1176–1184
SENASA (2016) http://www.senasa.gob.ar/senasa-comunica/noticias/se-recuerda-que-no-esta-permitido-el-uso-de-antibioticos-en-las-colmenas
Shimanuki, H. (1997) Bacteria. In: Flottum, K. (Ed.) Honey Bee Pests, Predators, and Disease, third ed. pp. 33–54, Medina, Ohio
Shukla, Y.N., Srivastava, A., Kumar, S., Kumar, S. (1999) Phytotoxic and antimicrobial constituents of Argyreia speciosa and Oenothera biennis. J. Ethnopharmacol. 67 (2), 241–245
Taguri, T., Tanaka, T., Kouno, I. (2006) Antibacterial spectrum of plant polyphenols and extracts depending upon hydroxyphenyl structure. Biol. Pharm. Bull. 29 (11), 2226–2235
Versalovic, J., Schneider, M., De Bruijn, F.J., Lupski, J.R. (1994) Genomic fingerprinting of bacteria using repetitive sequence-based polymerase chain reaction. Methods Mol. Cell. Biol. 5 (1), 25–40
Vianna Santos, R.C., Lopes, L.Q.S, dos Santos Alves, C.F., Fausto, V.P., Pizzutti, K., et al. (2014) Antimicrobial activity of tea tree oil nanoparticles against American and European foulbrood diseases agents. J. Asia Pac. Entomol. 17 (3), 343–347
Vico, T.A., Arce, V.B., Fangio, M.F., Gende, L.B., Bertran, C.A., Mártire, D.O., Churio, M.S. (2016) Two choices for the functionalization of silica nanoparticles with gallic acid: characterization of the nanomaterials and their antimicrobial activity against Paenibacillus larvae. J. Nanopart. Res. 18 (11), 348–360
Yah, C.S., Simate, G.S. (2015) Nanoparticles as potential new generation broad spectrum antimicrobial agents. DARU 23, 43 doi: https://doi.org/10.1186/s40199-015-0125-6
Yang, F.L., Li, X.G., Zhu, F., Lei, C.L. (2009) Structural characterization of nanoparticles loaded with garlic essential oil and their insecticidal activity against Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). J. Agric. Food. Chem. 57 (21), 10156–10162
Yilmaz, Y., Toledo, R.T. (2004) Major flavonoids in grape seeds and skins: antioxidant capacity of catechin, epicatechin, and gallic acid. J. Agric. Food. Chem. 52 (2), 255–260
Funding
This work was supported by CONICET, EXA Project No. 779/16 (UNMDP) and CONICET PIP Project No. 0804 to LG.
Author information
Authors and Affiliations
Contributions
AVB and MDO synthesized nanohybrids; MSN performed computer analyzes; ABS and MMP collaborated in antimicrobial assays; CMS read and corrected the manuscript; DN and GLB led the work.
Corresponding author
Additional information
Manuscript editor: Monique Gauthier
Bioactivité de l'acide gallique conjuguée à des nanoparticules de silice contrePaenibacillus larvaeet leur hôte, Apis mellifera
Apis mellifera / Paenibacillus larvae / Acide gallique / Nanohybride de silice
Bioaktivität von Gallussäure und konjugierten Silica-Nanopartikeln gegenüberPaenibacillus larvaeund gegenüber dem Wirt, der HonigbieneApis mellifera
Apis mellifera / Paenibacillus larvae / Gallussäure / Silica-Nanopartikel
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Domínguez, E., Moliné, M.P., Churio, M.S. et al. Bioactivity of gallic acid–conjugated silica nanoparticles against Paenibacillus larvae and their host, Apis mellifera honeybee. Apidologie 50, 616–631 (2019). https://doi.org/10.1007/s13592-019-00675-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13592-019-00675-y