Abstract
The comparison of social systems, particularly in closely related taxa, can be highly valuable to the understanding of social evolution. While much research has focused on the formation of hierarchies and eusocial organisation, it needs to be remembered that not all social systems are necessarily based on hierarchies. The allodapine bee Exoneurella tridentata is the only eusocial species within the entire subfamily Xylocopinae (Apidae) with discrete queen and worker morphology. Here, we show that a non-eusocial congener, Exoneurella eremophila, is casteless. Nest collection and dissection data show no evidence of hierarchies, and there were no per capita benefits to group nesting in terms of brood production in any collection period. The casteless behaviour exhibited by E. eremophila appears to be common among very diverse lineages of the bee tribe Allodapini, and as such represents an evolutionarily persistent behavioural strategy. We discuss likely ecological factors that may have driven the evolution of species lacking castes and a species with morphologically distinct castes from within a small monophyletic group—genus Exoneurella.
Similar content being viewed by others
1 Introduction
Eusociality is the most extreme form of social organisation, and the evolution of this trait often involves a major increase in behavioural complexity and interdependence of individual organisms (Szathmary and Maynard Smith 1995). Eusocial species have one or a few females who control the reproduction in the colony but are not totipotent, while the worker females are largely non-reproductive and are required to rear brood (Crespi and Yanega 1995; Dew et al. 2016). Evolutionary transitions to eusociality are the subject of a large body of research, but direct studies aimed at identifying how it has evolved are limited as most eusocial lineages have ancient origins, with no closely related extant non-eusocial taxa (Brady et al. 2006). This has led to an increasing recognition of the importance and power in studying those taxa that contain recent origins of eusociality, as well as more simple forms of organisation (Kocher and Paxton 2014; Rehan and Toth 2015). Traditionally, comparative studies have focused on societies with ‘primitive’ hierarchical organisation, perceived as potential precursory states leading to eusociality.
Not all social groups form hierarchies, with some societies appearing to form more-or-less egalitarian societies that can be regarded as ‘casteless’ (Wcislo and Tierney 2009; Dew et al. 2016). Hierarchies can manifest in different ways, but most are signified by one or more females getting greater control of reproduction (reproductive skew) and resources, or they may have a reduced share of the risky tasks performed for the colony, such as foraging. Casteless societies are colonies of two or more totipotent females that cooperate in the management of resources used for brood rearing; they exhibit no hierarchies, such that colony members are not constrained to specific behavioural roles such as reproduction, foraging, or guarding (Dew et al. 2016). Casteless behaviour is not to be confused with the term ‘communal’. While communal species may be casteless, and the term is commonly used to imply this, not all species labelled communal truly lack hierarchies (e.g. Sakagami and Maeta 1984; Kukuk et al. 1998; Ward and Kukuk 1998; Stark et al. 1990). The term communal has historically been used loosely, using the nest architecture as the basis to assume the behaviour of the colony, without evidence for the absence of hierarchies. Here, we use the term ‘casteless’ to emphasise taxa where statistical tests have identified no evidence of hierarchies. This facilitates a powerful comparison to taxa with hierarchies. Understanding the social biology of casteless species can provide insights into incipient stages of social evolution without assuming that social complexity evolved in a step-like manner through ever-increasing levels of hierarchical complexity (Lin and Michener 1972, Michener 1985).
Identifying casteless societies is not straightforward and requires careful analyses in order to distinguish lifetime fitness from temporal snapshots of nesting biology.
However, the number of social insects identified as casteless has been growing. Recent studies on facultatively social bees use statistical resampling techniques to compare traits (ovary size, body size and wing wear), of colony nestmates against null expectations (random pairs drawn from the population as a whole), in order to determine differentiation in reproductive skew and foraging effort. This is a powerful technique for capturing non-hierarchical structuring within social groups (Dew et al. 2016). Studies utilising this methodology have indicated a lack of castes in the Australian temperate colletid bee Amphylaeus morosus Smith (Spessa et al. 2000), the tropical Asian-Pacific allodapine bee Braunsapis puangensis Cockerell (da Silva et al. 2016) and Exoneurella setosa Houston from Australian semi-arid riparian regions (Dew et al. 2017).
The identification of casteless societies within a population of Exoneurella setosa (Dew et al. 2017) illuminates an interesting dichotomy in the social evolution of this genus. Its congener, E. tridentata Houston, is eusocial with reproductive queens and sterile workers that are morphologically differentiated (Hurst 2001; Houston 1976). Aside from the obligately eusocial E. tridentata, the remaining Exoneurella species are facultatively social—with populations consisting of both solitary nests with one adult female and social colonies with two or more adult females that cooperate in the management of resources used for brood rearing (Dew et al. 2016). Facultatively social Exoneurella species include E. lawsoni Rayment (Michener 1964), E. eremophila Houston (Hogendoorn et al. 2001) and the casteless E. setosa (Neville et al. 1998; Dew et al. 2017). It is unknown if casteless behaviour is unique to E. setosa or if it occurs in the other facultatively social Exoneurella as well. If castelessness is a common social trait of Exoneurella, this would have important implications for our understanding of social evolution.
The only previous study of E. eremophila examined populations in severely arid regions of central South Australia (Hogendoorn et al. 2001), and this suggested that social hierarchies might be absent, as determined by comparisons of body size between mated and unmated females in the population. However, further evidence for reproductive or task-based hierarchies was not explored, such as comparisons between the nestmate’s relative ovarian development and body size.
The aim of the current study is to determine the social structure of colonies of the allodapine bee E. eremophila in a riparian semi-arid population where it lives in sympatry with casteless colonies of E. setosa. Specifically, we tested the presence or absence of hierarchies in social groups, utilising statistical resampling techniques. It was predicted that there would be an absence of hierarchies in social groups, indicated by similarities in morphological traits relating to reproduction and foraging between colony members. We discuss the ecological factors that may have facilitated the evolution of castelessness and look at the prevalence of this behaviour across the allodapines as a whole.
2 Materials and methods
2.1 Sample collection
Collections were taken from Mildura, Victoria, Australia (34° 09′ 16.4″ S, 142° 09′ 23.9″ E) in areas of chenopod shrubland, within 2 km of the banks of the Murray River. There were three separate collection dates covering spring (11–13 October 2013), summer (21–23 January 2014) and autumn (12–14 April 2014). Whole nests were collected during early morning or late evening, when all colony members would be present in the nest, mostly from dead stems of annual Compositae plants in the genus Senecio. Entrances to the nests were taped to prevent bees escaping, and nests were placed in a cool box for transport to Flinders University for processing. We recorded nest census data including the number of adult females and males, type and number of brood, sex of pupae, nest length and any evidence of predation or parasitism. All nest contents were preserved in 100% ethanol. Adult females were later transferred to 70% ethanol to allow hydration of tissues to facilitate dissection. Numerical sex ratios (r = male pupae / total pupae) were calculated to compare sex allocation between social and solitary nests.
2.2 Dissections
Wing length was measured from the tip of the submarginal cell to the axillary sclerites. In allodapines, wing length is proportional to pupal weight (Schwarz 1986) and is an appropriate proxy for body size. Both forewings were scored for the number of nicks on the outer edge. In badly worn wings, individual nicks are impossible to discern, so these were given a score of > 20. A metric of ovarian size was calculated for each female by summing the length of the three largest oocytes as a measure of reproductive investment (Cini et al. 2013; Schwarz 1986). Individual spermathecae were observed for evidence of insemination, indicated by opalescence or transparency when not inseminated (Schwarz 1986). All data generated in this study are available in the figshare repository (DOI: https://doi.org/10.6084/m9.figshare.5439496; DOI: https://doi.org/10.6084/m9.figshare.5442349).
2.3 Statistical analyses
Data were analysed in SPSS v19.0.1 and R v3.1.0 (R Development Core Team 2015). Our variables of interest did not match normal distributions, so non-parametric analyses were used to compare metrics of ovary size, body size and wing wear among nestmates. Per capita brood production (total brood / adult females per nest) was compared between social and solitary nests as a measure of colony rearing efficiency (Michener 1964, 1974; Tierney et al. 1997; Schwarz et al. 2007). Each social nest has multiple individuals, which could create pseudoreplication problems when compared to solitary individuals (solitary nests—summer N = 19, autumn N = 21; social nests—summer N = 35, autumn N = 68). To address these potential problems, these samples were down weighted by their colony size as a fraction of the maximum colony size out of the dissected nests for that season. The results from the weighted and unweighted tests were the same.
Metrics of ovary size, body size and wing wear were further compared between nestmates and simulated random pairs of females in the population, using Monte Carlo resampling procedures (Spessa et al. 2000; Rehan et al. 2009; Tierney and Schwarz 2009, Tierney et al. 2013; da Silva et al. 2016; Dew et al. 2016). These analyses determined whether actual nestmates differed from each other in key traits, such as body and ovary size, more than would be expected from random pairings of non-nestmate or solitary nesting females. In theory, consistently larger differences in ovary size, body size or wing wear between nestmates, relative to random couplings, should be indicative of hierarchical nesting (e.g. Spessa and Schwarz 2000). Females in two-female nests were ranked 1 or 2 (largest or smallest) for each morphometric measured. We calculated the empirical mean difference between first- and second-rank females for each morphometric. We then simulated random pairs from a pool of all the two-female nesting individuals (with sample replacement) to get ‘expected’ nestmate differences. This was replicated 1000 times to generate null distributions for each morphometric. The empirical mean differences were then compared with the null distributions.
These analyses were then repeated using a pool of solitary nesting females from which ‘virtual’ pairs were drawn. Our rationale here is that individuals in a casteless nest should be performing all tasks (or a portion of all tasks), in the same way that a solitary female would, but in a shared space. Therefore, we would not expect empirical differences in morphometrics to differ from expected virtual pairs of randomly sampled solitary nesting females.
3 Results
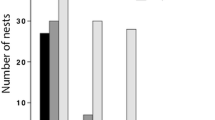
A total of 238 nests containing adult females were collected during summer (N = 39) and autumn (N = 195). Only four nests were found in spring and were excluded from statistical comparisons due to the low sample size. The modal colony size in both samples was one female per nest (Figure 1), but group nesting was more prevalent in autumn. During summer, the mean colony size was 1.41 with a maximum of three females per nest, and 33% of the nests contained more than one female. In autumn, the mean colony size rose to 2.46 with a maximum of nine females per nest, and the proportion of social nests was 61%, which likely represents the recruitment of recently emerged adults that will disperse in spring.
3.1 Morphometrics of solitary versus social nesting females
Females were regarded as social if there was more than one adult female in the nest, while solitary females were from nests with only one adult female. Solitary females were compared to those in social nests to get a baseline comparison of females choosing either nesting strategy. Dissections were performed on 143 individuals from 80 randomly sampled nests. The dissected samples included 40 solitary females and 103 females from social nests. There were seasonal differences in ovarian development, with females possessing larger ovaries in summer than in autumn (Mann-Whitney, U = 3953, P < 0.001); hence, all subsequent analyses were performed separately for each collection sample.
Mating behaviour was inferred from insemination status. The proportion of solitary and social females that had mated was not different in either summer (chi-square Χ 2 1 = 1.384, P = 0.222) or autumn (chi-square Χ 2 1 = 3.017, P = 0.082; Fig. 2). During summer, solitary and social females did not significantly differ in ovary size (Mann-Whitney U = 262, P = 0.49), wing length (U = 328, P = 0.94) or wing wear (U = 286, P = 0.35). However, in autumn, social females did exhibit significantly larger ovaries (0.58 mm ± 0.026 s.e.) than solitary females (0.37 mm ± 0.046 s.e.; U = 343, P = 0.001). Social females also had on average larger body sizes (2.37 mm ± 0.014 s.e.) than solitary females (2.25 mm ± 0.020 s.e.; U = 321, P < 0.001), while solitary females had more worn wings (mean wing nicks = 3.6 ± 1.32 s.e.) than their social counterparts (0.99 ± 0.37 s.e.; U = 914, P = 0.016).
In summer, social and solitary females did not have significantly different ovary sizes, body sizes or wing wear (U tests, P ≥ 0.15 for all three tests). But social females in autumn were found to have significantly greater ovary sizes (U = 3423, P < 0.001) and body sizes (U = 2823, P < 0.001), corroborating the unweighted analyses. Solitary females in autumn were likewise found to have more wing wear than social females (U = 4973, P < 0.001).
3.2 Social group structure
Next, we compared members of social nests, to determine whether within-nest social differentiation was present. The proportion of inseminated social females did not differ in summer and autumn (76 and 78% respectively, Fig. 2). In summer, mated social females had larger ovaries (mean 2.28 mm ± 0.15 s.e.) than unmated social females (mean 0.82 mm ± 0.14 s.e.; U = 8.5, P < 0.001). Mated and unmated females did not have significantly different ovary sizes in autumn (U = 197, P = 0.167). This equivalence in ovary size is due to decreased ovarian development in mated females at this time, with ovary size of mated females in autumn (0.06 mm ± 0.027 s.e.) showing a large drop from that of mated females in summer (mean 2.28 mm ± 0.15 s.e.; U = 1027, P < 0.001). Wing length and wing wear showed no significant pattern with insemination in either season (wing length U tests P ≥ 0.36, wing wear U tests P ≥ 0.20).
Individuals from two-female nests only (due to the small sample size of larger colonies) were then compared for evidence of hierarchies in both summer and autumn. Females in each nestmate pair were ranked based on ovary size, body size and wing wear separately. Females ranked by ovary size did not have significantly different body sizes (both U tests, P ≥ 0.73) or wing wear (both U tests, P ≥ 0.21) in either season. Similarly, females ranked by body size did not have significantly different ovary sizes (both U tests, P ≥ 0.77) or wing wear (both U tests, P ≥ 0.36). Again, when ranked by wing wear, nestmates did not exhibit significantly different body sizes (both U tests, P ≥ 0.16) or ovary sizes (both U tests, P ≥ 0.47).
3.3 Monte Carlo simulations
Monte Carlo simulations were used to further explore patterns in morphometrics among twos-female nests. Only the summer collection sample was used for simulated analyses, as this period represents the peak of reproduction and brood rearing in our samples and should allow us to capture any patterns in reproductive skew among nestmates, were they to exist. Random nestmate pairs were simulated 1000 times in two different ways: social pool, from the pooled two-female nests (N = 13), and solitary pool, from the pooled solitary females (N = 20). We did this because social nests could have non-random morphometric compositions not detected in the previous comparisons of social and solitary individuals (see section ‘3.1’).
Results from social pool (two-female nests) indicate that 97% of the 1000 simulated pairs showed greater differences in ovary size than the mean difference between empirical nestmate pairs of 0.59 mm (Fig. 3). Therefore, individuals in two-female nests were more similar than expected from the simulated distribution. When this analysis was repeated with simulated pairs drawn from the solitary pool (solitary nests), 84% had greater differences in ovary size than the observed mean difference, strongly suggesting an absence of reproductive castes. This indicates that ovary size differences in social pairs are less than one would expect based on random assignment of females to virtual nests. For body size, 58% (social pool) and 34% (solitary pool) of the simulated pairs had greater differences than the observed social mean, again providing no evidence of hierarchies. Similar results were obtained for wing wear with 32% (social pool) and 69% (solitary pool) of the simulated distribution having greater differences than the mean difference between observed pairs.
Frequency of differences in ovary size (a), body size (b) and wing wear (c) between virtual nestmate pairs. Pairs were randomly drawn from summer social nests (pool a) in a Monte Carlo resampling procedure. Grey bars indicate the proportion of virtual pairs exhibiting greater differences than the observed mean derived from actual nest pairs.
3.4 Brood production
The majority of nests had brood present in both summer (69%) and autumn (72%), though the composition of the brood varied across seasons (Figure 4). Of the four nests collected in spring, three had brood, consisting of both eggs and larvae, indicating that brood production started as early as late September. Eggs, larvae and pupae were present in summer. In autumn, only one nest was found to have an egg, with larvae and/or pupae present in all other nests that were rearing brood. There were a total of three nests (one solitary and two social) found with brood parasitised by encyrtid wasps, all in the autumn sample.
Per capita brood production (PCBP) was compared between solitary and two-female nests with Mann-Whitney U tests (sample sizes of larger social nests were too small for meaningful comparison). For both summer and autumn, there were no significant differences in PCBP between social and solitary nests (U = 97, P = 0.21; U = 1353, P = 0.055 respectively).
Sex allocation did not vary significantly between seasons (U = 1148, P = 0.32), with an overall mean sex ratio of 0.45 ± 0.020 s.e. Sex ratios in social and solitary nests did not vary in summer (U = 45, P = 0.72). But during autumn, sex ratios were significantly more female biased in solitary nests (r = 0.29) than social nests (r = 0.52; U = 947, P < 0.001).
4 Discussion
Our results provide strong evidence that social colonies of E. eremophila are casteless. There was no skew between social nestmate pairs in insemination status, ovary size, body size or wing wear other than expected from randomly assembled ‘virtual nestmates’. Females in social nests were in fact more similar in ovary size than that expected from randomly assembled pairs (Figure 3). There are a number of possible explanations for this. Firstly, females may co-found nests and represent breeding cohorts at similar reproductive stages, although nest co-founding is not known to occur in any species of Exoneurella. Second, environmental resources may standardise ovarian development across the population, such as the availability of protein resources from pollen. Third, female nestmates may be highly related and ovarian development is genetically conserved.
In addition to a lack of castes, we also found only minor benefits to group nesting. One third of nests are social during summer (the peak brood-rearing period), so we might expect fitness benefits to social nesting; however, there were no differences in PCBP between social and solitary nests. There may be minor benefits associated with social nesting as the brood-rearing season comes to a close because autumn social females had larger ovary sizes than solitary nesting females (Figure 3). A similar result was found for the sympatric species E. setosa (Dew et al. 2017). Social individuals may experience a slightly extended reproductive period compared to solitary females, perhaps due to co-operative nest defence. Social females in autumn also had larger body sizes, which could be due to better provisioning of newly eclosed adults in social nests. Wing wear was reduced in social females compared to their solitary counterparts in autumn, although this result could be due to the emergence of recently eclosed adult brood.
Sex ratios were considerably male biased in social colonies at the end of the brood-rearing season. Such seasonal variation in sex ratio bias is consistent with species that exhibit caste differentiation, whereby initial broods are female biased to increase the potential for alloparental care (worker castes) to arise. Similar results were discovered in a study of facultatively social E. lawsoni from subtropical tableland savannahs of eastern Australia (Michener 1964). It is therefore speculative as to whether (i) non-eusocial species of Exoneurella derive from a eusocial common ancestor and have lost worker-like behaviour to become casteless (per Michener) or whether (ii) these casteless Exoneurella species (Michener 1964; Dew et al. 2016, 2017) have in fact set social preconditions allowing the only truly eusocial allodapine species to arise.
4.1 Sociality and ecology of Exoneurella
Fitness gains via improved brood-rearing efficiency in social nests are thought to represent one of the main selective agents for the formation of insect societies generally and are commonly found among allodapine bees (e.g. Michener 1974; Schwarz et al. 1998; Tierney et al. 1997, 2000, 2002; Joyce and Schwarz 2006; Thompson and Schwarz 2006). However, social evolution in the genus Exoneurella does not appear to be influenced by brood-rearing efficiency, as none of the Exoneurella studied to date show improved PCBP with increasing colony size, not even in the eusocial species E. tridentata (Dew et al. 2017; Hurst 2001; Michener 1974).
Benefits to group living may consist of improved nest guarding, enabling temporally extended brood rearing deeper into autumn. Intracolony relatedness is exceedingly high in eusocial E. tridentata (r = 0.75; Hurst 2001) and is presumed to be high in E. setosa and E. eremophila, with mother-daughter or sister-sister associations most likely developing within natal nests (Hogendoorn et al. 2001; Neville et al. 1998). Therefore, when coupled with high relatedness, even minimal benefits to group living may prove sufficient selective pressure to promote social nesting regardless of the structure of the resultant social organisation.
While even meagre benefits to group living may promote sociality within Exoneurella generally, evolution of variant social structures may have evolved in response to the relative costs of dispersal. Differences in both the availability and longevity of stem nesting substrate appear to be associated with large differences in dispersal opportunity, and it is therefore plausible that nest substrates may have been key determinants in social behaviour. The evolution of eusociality in E. tridentata has been linked to extreme nest site limitations (Dew et al. 2012), because of their reliance on pre-formed burrows in two species of hardwood trees, for which there is competition from a variety of other arthropods (Dew and Schwarz 2013). Dispersal from the natal nest is therefore likely to be very risky because the likelihood of finding suitable nesting habitats is very low. We argue that this is likely to facilitate the evolution of eusocial organisation via the formation of long-term colonies that are further enabled by the durability of the hardwood nesting substrate.
A similar dispersal-linked hypothesis—the aridity food distribution hypothesis—has also been proposed to explain the variation in social behaviour of different mole rat species (Jarvis et al. 2005; Spinks et al. 2000; Sichilima et al. 2008). Mathematical modelling suggests that nest-site limitations or similarly high dispersal risks may be sufficient to promote eusocial evolution on their own, even in the absence of brood production benefits (Avila and Fromhage 2015).
In comparison, the casteless Exoneurella species are able to nest in a wide variety of plant substrates that are in ample supply and renewed annually. In this sense, there are few limitations to dispersal; however, the nesting substrates are of an ephemeral durability and less likely to persist across multiple seasons. Indeed, the high level of solitary nesting reported in these species indicates that dispersal from the natal nest is not restricted, nor is it an overwhelming risk (Michener 1964; Neville et al. 1998; Hogendoorn et al. 2001; Dew et al. 2017). Minimal restrictions to dispersal have also been noted in casteless Braunsapis puangensis and Amphylaeus morosus (da Silva et al. 2016; Spessa et al. 2000). It appears that low barriers to dispersal, with small benefits to social living, are key factors in casteless society formation in Exoneurella and possibly other casteless groups.
4.2 Evolution of casteless societies among Apiformes
Casteless behaviour occurs in diverse allodapine genera and is potentially ancestral to the allodapine bees as a whole. Reconstruction of the most recent common ancestor of the Allodapini indicates social groups were present but true castes were not; rather, there was temporal reproductive skew due to inter-generational delay in reproduction (Schwarz et al. 2011; Rehan et al. 2012). Notably, reproductives in ancestral allodapines were also foragers, permitting the possibility of primitive casteless societies. The casteless behaviour of Macrogalea, which forms the sister clade to all other allodapines, likewise suggests that castelessness has ancestral origins (Tierney et al. 2002; Thompson and Schwarz 2006; Butler et al., pers. comm.). But whether ancestral reproductive queues are viewed as casteless or not hinges on whether individuals attained reproductive roles later in life and thereby had equal lifetime reproductive opportunities. This is difficult to assess (see Dew et al. 2016), and it is quite possible that some ‘waiting’ females did not attain full reproductive status, forming rudimentary hierarchies. Or maybe this natal philopatry simply allowed flexibility in social structure, enabling the diversity of social forms seen in the Allodapini today.
If the allodapines are ancestrally casteless, then this trait may have simply been retained in most Exoneurella. Currently, however, the behaviour at the root of Exoneurella has not been established. The most basal Exoneurella is an undescribed species from Western Australia (Chenoweth and Schwarz 2011). The social behaviour of this species is unknown, but it does not have dimorphic females like those of E. tridentata (R. Dew, pers. obs.). The possibility of a socially hierarchical common ancestor to the genus Exoneurella cannot be ruled out until the social behaviour of this western species is known (Chenoweth and Schwarz 2011).
While the ancestral condition for Exoneurella is still ambiguous, a loss of hierarchies to casteless behaviour is known for the allodapine Braunsapis puangensis, which lost facultative castes upon its recent introduction to Fiji from India (da Silva et al. 2016). Similarly, the casteless behaviour of Euglossa hyacintha Dressler (Apidae: Euglossini) may represent a loss of hierarchies and thereby a reversion to castelessness (Cardinal and Danforth 2011; Soucy et al. 2003). The colletid bee Amphylaeus morosus (Colletidae: Hylaeinae), however, evolved casteless behaviour from solitary living, as the vast majority of colletids are solitary (Spessa et al. 2000).
The number of casteless lineages now identified and their inherent similarity to communal nesting lineages (reviewed by Dew et al. 2016) suggest that the absence of castes in social insect organisation has been a persistent and successful nesting strategy over significant periods of evolutionary time. This highlights that casteless and eusocial societies may simply represent an alternate means of achieving the same end—the establishment of a secure abode in which to rear brood (see Wcislo and Tierney 2009).
So while the broader comparative evidence clearly shows that castelessness is not just a transitional behavioural state on a directional trajectory towards a hierarchical eusocial optimum, the evolutionary history of the genus Exoneurella remains opaque. Do non-eusocial Exoneurella represent the loss of castes from a eusocial common ancestor (resembling E. tridentata colonies), or did an absence of castes accompanied by (i) severe limitations to natal-nest dispersal and (ii) a highly durable nesting substrate facilitate the evolution of extreme social hierarchies? If the latter is true, then extrinsic chance events have created a platform for the evolution of morphologically distinct behavioural castes and the development of truly eusocial colony organisation. In short, did these bees need to lose castes in order to comprehensively develop them?
References
Avila, P., Fromhage, L. (2015) No synergy needed: ecological constraints favor the evolution of eusociality. Am. Nat. 186 (1), 31–40
Brady, S.G., Sipes, S., Pearson, A., Danforth, B.N. (2006) Recent and simultaneous origins of eusociality in halictid bees. Proc. R Soc. B. https://doi.org/10.1098/rspb.2006.3496
Cardinal, S., Danforth, B.N. (2011) The antiquity and evolutionary history of social behavior in bees. PLoS ONE. 6 (6), e21086. https://doi.org/10.1371/journal.pone.0021086
Chenoweth, L.B., Schwarz, M.P. (2011) Biogeographical origins and diversification of the exoneurine allodapine bees of Australia (Hymenoptera, Apidae). J. Biogeogr. 38, 1471–1483. https://doi.org/10.1111/j.1365-2699.2011.02488.x
Cini, A., Meconcelli, S., Cervo, R. (2013) Ovarian indexes as indicators of reproductive investment and egg-laying activity in social insects: a comparison among methods. Insectes Soc. 60, 393–402. https://doi.org/10.1007/s00040-013-0305-7
Crespi, B.J., Yanega, D. (1995) The definition of eusociality. Behav. Ecol. 6 (1), 109-115
da Silva, C.R.B., Stevens, M., Schwarz, M.P. (2016) Casteless societies evolve from hierarchical/eusocial systems: evidence from an allodapine bee. Insectes Soc. 63, 67–78. https://doi.org/10.1007/s00040-015-0436-0
Dew, R.M., Schwarz, M.P. (2013) Distribution of the native South Australian bee Exoneurella tridentata in Western Myall (Acacia papyrocarpa) woodlands. S. Aust. Nat. 87 (2), 70–74.
Dew, R.M., Rehan, S.M., Tierney, S.M., Chenoweth, L.B., Schwarz, M.P. (2012) A single origin of large colony size in allodapine bees suggests threshold event among 50 million years of evolutionary tinkering. Insectes Soc. 59, 207–214. https://doi.org/10.1007/s00040-011-0206-6
Dew, R.M., Tierney, S.M., Schwarz, M.P. (2016) Social evolution and casteless societies: needs for new terminology and a new evolutionary focus. Insectes Soc. 63, 5–14. https://doi.org/10.1007/s00040-015-0435-1
Dew, R.M., Tierney, S.M., Schwarz, M.P. (2017) Lack of ovarian skew in an allodapine bee and the evolution of casteless social behavior. Ethol. Ecol. Evol. https://doi.org/10.1080/03949370.2017.1313784
Hogendoorn, K., Watiniasih, N.L., Schwarz, M.P. (2001) Extended alloparental care in the almost solitary bee Exoneurella eremophila (Hymenoptera: Apidae). Behav. Ecol. Sociobiol. 50, 275–282. https://doi.org/10.1007/s002650100357
Houston, T.F. (1976) New Australian allodapine bees (subgenus Exoneurella Michener) and their immatures (Hymenoptera: Anthophoridae). Trans. R. Soc. S. Aust. 100, 15–28
Hurst, P.S. (2001) Social biology of Exoneurella tridentata, an allodapine with morphological castes and perennial colonies. PhD thesis, School of Biological Sciences, The Flinders University of South Australia, Adelaide, Australia
Jarvis, J.U.M., O’Riain, J., Bennett, N.C., Sherman, P.W. (2005) Mammalian eusociality: a family affair. Trends Ecol. Evol., 9 (2), 47–51
Joyce, N.C., Schwarz, M.P. (2006) Sociality in the Australian allodapine bee Brevineura elongata: small colony sizes despite large benefits to group living. J. Insect. Behav. 19 (1), 45–61. https://doi.org/10.1007/s10905-005-9004-1
Kocher, S.D., Paxton, R.J. (2014) Comparative methods offer powerful insights into social evolution in bees. Apidologie, 45, 289–305. https://doi.org/10.1007/s13592-014-0268-3
Kukuk, P.F., Ward, S.A., Jozwiak, A. (1998) Mutualistic benefits generate an unequal distribution of risky activities among unrelated group members. Naturwissenschaften 85, 445–449. https://doi.org/10.1007/s001140050528
Lin, N., Michener, C.D. (1972) Evolution of sociality in insects. Q. Rev. Biol. 47 (2), 131–159
Michener, C.D. (1964) The bionomics of Exoneurella, a solitary relative of Exoneura (Hymenoptera: Apoidea: Ceratini). Pac. Insect. 6, 411–426
Michener, C.D. (1974) The social behavior of the bees. The Belknap Press of Harvard University Press, Cambridge
Michener, C.D. (1985). From solitary to eusocial - need there be a series of intervening species. Fortschr. Zool. 31, 293–305.
Neville, T., Schwarz, M.P., Tierney, S.M. (1998) Biology of a weakly social bee, Exoneura (Exoneurella) setosa (Hymenoptera: Apidae) and implications for social evolution in Australian allodapine bees. Aust. J. Zool. 46, 221–234
R Development Core Team (2015). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3–900051–07-0. [online] http://www.Rproject.org/. (accessed October 2015)
Rehan, S.M., Toth, A.L. (2015) Climbing the social ladder: the molecular evolution of sociality. Trends Ecol. Evol., 30(7): 426–433. https://doi.org/10.1016/j.tree.2015.05.004
Rehan, S.M., Richards, M.H., Schwarz, M.P. (2009) Evidence of social nesting in the Ceratina of Borneo (Hymenoptera: Apidae). J. Kansas. Entomol. Soc. 82, 194–209. https://doi.org/10.2317/JKES809.22.1
Rehan, S.M., Leys, R., Schwarz, M.P. (2012). A mid-cretaceous origin of sociality in xylocopine bees with only two origins of true worker castes indicates severe barriers to eusociality. PLoS ONE, 7(4) e34690. https://doi.org/10.1371/journal.pone.0034690
Sakagami, S.F., Maeta, Y. (1984) Multifemale nests and rudimentary castes in the normally solitary bee Ceratina japonica (Hymenoptera, Xylocopinae). J. Kansas Entomol. Soc. 57, 639–656
Schwarz, M.P. (1986) Persistent multi-female nests in an Australian allodapine bee, Exoneura bicolor (Hymenoptera, Anthophoridae). Insectes Soc. 33, 258–277. https://doi.org/10.1007/BF02224245
Schwarz, M.P., Bull, N.J., Hogendoorn, K. (1998). Evolution of sociality in the allodapine bees: a review of sex allocation, ecology and evolution. Insectes Soc. 45, 349–368
Schwarz, M.P., Richards, M.H., Danforth, B.N. (2007) Changing paradigms in insect social evolution: insights from halictine and allodapine bees. Annu. Rev. Entomol. 52, 127–150. https://doi.org/10.1146/annurev.ento.51.110104.150950
Schwarz, M.P., Tierney, S.M., Rehan, S.M., Chenoweth, L.B., Cooper, S.J. (2011) The evolution of eusociality in allodapine bees: workers began by waiting. Biol. Lett. 7, 277–280. https://doi.org/10.1098/rsbl.2010.0757
Sichilima, A.M., Bennett, N.C., Faulkes, C.G., Le Comber, S.C. (2008) Evolution of African mole-rat sociality: burrow architecture, rainfall and foraging in colonies of the cooperatively breeding Fukomys mechowii. J. Zool. 275, 276–282
Soucy, S.L., Giray, T., Roubik, D.W. (2003) Solitary and group nesting in the orchid bee Euglossa hyacinthina (Hymenoptera, Apidae). Insectes Soc. 50, 248–255. https://doi.org/10.1007/s00040-003-0670-8
Spessa, A., Schwarz, P., Adams, M. (2000) Sociality in Amphylaeus morosus (Hymenoptera : Colletidae : Hylaeinae). Ann. Entomol. Soc. Am. 93, 684–692
Spinks, A.C., Jarvis, J.U.M., Bennett, N.C. (2000) Comparative patterns of philopatry and dispersal in two common mole-rat populations: implications for the evolution of mole-rat sociality. J. Anim. Ecol. 69, 224–234
Stark, R.E., Hefetz, A., Gerling, D., Velthuis, H.H.W. (1990) Reproductive competition involving oophagy in the socially nesting bee Xylocopa sulcatipes. Naturwissenschaften 77, 38–40. https://doi.org/10.1007/BF01131797
Szathmary, E., Maynard Smith, J. (1995) The major evolutionary transitions. Nature 374, 227–232
Thompson, S., Schwarz, M.P. (2006) Cooperative nesting and complex female-biased sex allocation in a tropical allodapine bee. Biol. J. Linn. Soc. 89, 355–364
Tierney, S.M., Schwarz, M.P. (2009) Reproductive hierarchies in the African allodapine bee Allodapula dichroa (Apidae; Xylocopinae) and ancestral forms of sociality. Biol. J. Linn. Soc. 97, 520–530
Tierney, S.M., Schwarz, M.P., Adams, M. (1997) Social behaviour in an Australian allodapine bee Exoneura (Brevineura) xanthoclypeata (Hymenoptera : Apidae). Aust. J. Zool. 45, 385–398. https://doi.org/10.1071/ZO97022
Tierney, S.M., Cronin, A.L., Loussert, N., Schwarz, M.P. (2000) The biology of Brevineura froggatti and phylogenetic conservatism in Australian allodapine bees (Apidae, Allodapini). Insectes Soc. 47, 96–97
Tierney, S.M., Schwarz, M.P., Neville, T., Schwarz, P.M. (2002) Sociality in the phylogenetically basal allodapine bee genus Macrogalea (Apidae: Xylocopinae): implications for social evolution in the tribe Allodapini. Biol. J. Linn. Soc. 76, 211–224
Tierney, S.M., Fischer, C.N., Rehan, S.M., Kapheim, K.M., Wcislo, W.T. (2013) Frequency of social nesting in the sweat bee Megalopta genalis (Halictidae) does not vary across a rainfall gradient, despite disparity in brood production and body size. Insectes Soc. 60, 163–172. https://doi.org/10.1007/s00040-012-0280-4
Ward, S.A., Kukuk, P.F. (1998) Context-dependent behavior and the benefits of communal nesting. Am. Soc. Nat. 152, 249–263
Wcislo, W.T., Tierney, S.M. (2009) The evolution of communal behavior in bees and wasps: an alternative to eusociality, in: Gadau, J. and Fewell, J. (Eds.), Organization of Insect Societies from genome to sociocomplexity. Harvard University Press, Cambridge, pp. 148–169
Acknowledgements
We thank O. Davies, R. Kittel and N. Shokri Bousjein for assistance with fieldwork.
Funding
This research was funded by a Holsworth Wildlife Research Endowment, a Sir Mark Mitchell Foundation grant and a Lirabenda Endowment fund grant to R. Dew.
Author information
Authors and Affiliations
Contributions
MS and RD designed the experiment and performed field work with ST. RD did all lab work and analyses. RD wrote the paper with revisions by MS, ST and MG who all read and approved the final version.
Corresponding author
Additional information
Handling editor: David Tarpy
Comportement de société sans caste dans les groupes sociaux de l’abeille Exoneurella eremophila
sans caste / Allodapini / comportement social / espèce eusociale / limitation des sites de nid
Kastenloses Verhalten in sozialen Gruppen der Biene Exoneurella eremophila
Kastenlos / Allodapine Bienen / Sozialverhalten / eusozial / Nistplatz-Beschränkungen
Rights and permissions
About this article
Cite this article
Dew, R., Tierney, S., Gardner, M. et al. Casteless behaviour in social groups of the bee Exoneurella eremophila . Apidologie 49, 265–275 (2018). https://doi.org/10.1007/s13592-017-0550-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13592-017-0550-2