Abstract
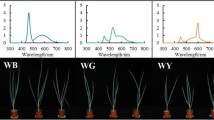
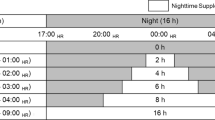
Artemisia princeps (Ganghwa wormwood) is a medicinal plant that produces two major flavonoids, eupatilin and jaceosidin, which are used in the treatment of gastritis and peptic ulcers. A. princeps is primarily field cultivated, which has some drawbacks, including only one cultivation period per year and variations in flavonoid production due to environmental changes. The objective of this study was to analyze the effects of seasonal light variation and artificial light treatments on the growth and flavonoid production of A. princeps grown in greenhouses for year-round production. The plants were cultivated and harvested nine times in one year under natural seasonal light conditions in greenhouses. During the winter growth period (when natural light is substantially lower), four artificial light treatments were applied during two cultivation periods, from September 2016 to January 2017: supplemental light, night interruption, low light, and low light with night interruption. The plants grown under the natural light condition in greenhouses were used as a control. After harvest, the growth of the plants was measured, and the contents of eupatilin and jaceosidin were determined. The plants had the highest biomass when the accumulated radiation and duration were highest. The growth and flavonoid production were significantly associated with accumulated radiation and light duration. The supplemental light and night interruption treatments resulted in significantly higher biomass and flavonoid production, with the night interruption treatment requiring less energy input than the supplemental light treatment. Therefore, for consistent biomass and flavonoid production of A. princeps, a night interruption treatment is suggested in greenhouse cultivation during low irradiation and short days (less than 13 h).







Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Ahn JB, Hur JN, Jung HG, Park JH (2012) Study on the growth environment of ‘Gangwha-mugwort’ through the climatological characteristic analysis of Gangwha region. Kor J Agric For Meteorol 14:71–78. https://doi.org/10.5532/KJAFM.2012.14.2.071
Anand U, Jacobo-Herrera N, Altemimi A, Lakhssassi N (2019) A comprehensive review on medicinal plants as antimicrobial therapeutics: potential avenues of biocompatible drug discovery. Metabolites 9:258. https://doi.org/10.3390/metabo9110258
Behrenfeld MJ, Prasil O, Babin M, Bruyant F (2004) In search of a physiological basis for covariations in light-limited and light-saturated photosynthesis. J Phycol 40:4–25. https://doi.org/10.1046/j.1529-8817.2004.03083.x
Boardman NK (1977) Comparative photosynthesis of sun and shade plants. Annu Rev Plant Physiol 28:355–377. https://doi.org/10.1146/annurev.pp.28.060177.002035
Choi SR, You DH, Kim JY, Park CB, Ryu J, Kim DH, Eun JS (2008) Antioxidant and antimicrobial activities of Artemisia capillaris Thunberg. Kor J Med Crop Sci 16:112–117
Dorais M, Papadopoulos AP, Luo X, Leonhart S, Gosselin A, Pedneault K, Angers P, Gaudreau L (2001) Soilless greenhouse production of medicinal plants in North Eastern Canada. Acta Hortic 554:297–304. https://doi.org/10.17660/ActaHortic.2001.554.32
Elsayed AI, El-Hamahmy MAM, Rafudeen MS, Mohamed AH, Omar AA (2019) The impact of drought stress on antioxidant responses and accumulation of flavonolignans in milk thistle (Silybum marianum (l.) gaertn). Plants 8:611. https://doi.org/10.3390/plants8120611
Ferreira JF, Simon JE, Janick J (1995) Developmental studies of Artemisia annua. Flowering and artemisinin production under greenhouse and field conditions. Planta Med 61:167–170. https://doi.org/10.1055/s-2006-958040
Fonseca JM, Rushing JW, Rajapakse NC, Thomas RL, Riley MB (2006) Potential implications of medicinal plant production in controlled environments: the case of feverfew (Tanacetum parthenium). HortScience 41:531–535. https://doi.org/10.21273/HORTSCI.41.3.531
Gonzalez-Coloma A, Bailen M, Diaz CE, Fraga BM, Martínez-Díaz R, Zuñiga GE, Contreras RA, Cabrera R, Burillo J (2012) Major components of Spanish cultivated Artemisia absinthium populations: Antifeedant, antiparasitic, and antioxidant effects. Ind Crop Prod 37:401–407. https://doi.org/10.1016/j.indcrop.2011.12.025
Graham IA, Czechowski T, Rinaldi M, Famodimu M, Van Veelen M, Larson T, Horrocks P (2019) Flavonoid versus artemisinin anti-malarial activity in Artemisia annua whole-leaf extracts. Front Plant Sci 10:984. https://doi.org/10.3389/fpls.2019.00984
Höft M, Verpoorte R, Beck E (1996) Growth and alkaloid contents in leaves of Tabernaemontana pachysiphon Stapf (Apocynaceae) as influenced by light intensity, water, and nutrient supply. Oecologia 107:160–169
Jaakola L, Hohtola A (2010) Effect of latitude on flavonoid biosynthesis in plants. Plant Cell Environ 33:1239–1247. https://doi.org/10.1111/j.1365-3040.2010.02154.x
Jelodar NB, Bhatt A, Mohamed K, Keng CL (2014) New cultivation approaches of Artemisia annua L. for a sustainable production of the antimalarial drug artemisinin. J Med Plant Res 8:441–447. https://doi.org/10.5897/JMPR11.1053
Kang WH, Han Z, Lee SJ, Shin JH, Ahn TI, Lee JY, Son JE (2018) Analysis of year-round cultivation characteristics of Artemisia princeps in greenhouse and enhancement of eupathilin content by environmental stress. Protected Hort Plant Fac 27:94–101. https://doi.org/10.12791/KSBEC.2018.27.1.94
Kim YJ, Lee JH, Kim SJ (2013) Cultivation characteristics and flavonoid contents of wormwood (Artemisia montana Pamp.). J Agric Chem Environ 2:117–122. https://doi.org/10.4236/jacen.2013.24017
Kitayama M, Nguyen DTP, Lu N, Takagaki M (2019) Effect of light quality on physiological disorder, growth, and secondary metabolite content of water spinach (Ipomoea aquatica Forsk) cultivated in a closed-type plant production system. Hortic Sci Technol 37:206–218. https://doi.org/10.12972/kjhst.20190020
Konsin M, Voipio I, Palonen P (2001) Influence of photoperiod and duration of short-day treatment on vegetative growth and flowering of strawberry (Fragaria × ananassa Duch.). J Hortic Sci Biotechnol 76:77–82. https://doi.org/10.1080/14620316.2001.11511330
Lee JW, Kang WH, Park KS, Son JE (2017) Spectral dependence of electrical energy-based photosynthetic efficiency at single leaf and canopy levels in green- and red-leaf lettuces. Hortic Environ Biotechnol 58:111–118. https://doi.org/10.1007/s13580-017-0154-9
Lee JW, Son KH, Lee JH, Kim YJ, Oh MM (2019) Growth and biochemical responses of ice plant irradiated by various visible light spectra in plant factories. Hortic Sci Technol 37:598–608. https://doi.org/10.7235/HORT.20190060
Lee SH, Bae EA, Park EK, Shin YW, Baek NI, Han EJ, Chung HG, Kim DH (2007) Inhibitory effect of eupatilin and jaceosidin isolated from Artemisia princeps in IgE-induced hypersensitivity. Int Immunopharmacol 7:1678–1684. https://doi.org/10.1016/j.intimp.2007.08.028
Martínez-Lüscher J, Morales F, Delrot S, Sánchez-Díaz M, Gomès E, Aguirreolea J, Pascual I (2015) Characterization of the adaptive response of grapevine (cv. Tempranillo) to UV-B radiation under water deficit conditions. Plant Sci 232:13–22. https://doi.org/10.1016/j.plantsci.2014.12.013
Meki MN, Ogoshi RM, Kiniry JR, Crow SE, Youkhana AH, Nakahata MH, Littlejohn K (2017) Performance evaluation of biomass sorghum in Hawaii and Texas. Ind Crop Prod 103:257–266. https://doi.org/10.1016/j.indcrop.2017.04.014
Mosaleeyanon K, Zobayed SMA, Afreen F, Kozai T (2005) Relationships between net photosynthetic rates and secondary metabolite concentrations in St. John’s wort. Plant Sci 169:523–531. https://doi.org/10.1016/j.plantsci.2005.05.002
Naß J, Efferth T (2018) The activity of Artemisia spp. and their constituents against Trypanosomiasis. Phytomedicine 47:184–191. https://doi.org/10.1016/j.phymed.2018.06.002
Oh SI, Lee JH, Lee AK (2019) Growth, antioxidant concentrations and activity in Sedum takesimense as affected by supplemental LED irradiation with light quality. Hortic Sci Technol 37:589–597. https://doi.org/10.7235/HORT.20190059
Oluwasemire KO, Odugbenro GO (2014) Solar radiation interception, dry matter production, and yield among different plant densities of Arachis spp. in Ibadan, Nigeria. Agric Sci 5:864. https://doi.org/10.4236/as.2014.510093
Ryu SN (2008) Environmental variation of available component in Mugwort (Artemisia princeps Pamp.). J Kor Soc Intl Agric 20:40–46
Ryu SN, Han SS, Yang JJ, Jeong HG, Kang SS (2005) Variation of eupatilin and jaceosidin content of mugwort. Kor J Crop Sci 50:204–207
Uehara A, Shimoda K, Murai Y, Iwashina T (2018) Flavonoid aglycones and glycosides from the leaves of some Japanese Artemisia Species. Nat Prod Commun 13:1934578X1801300510. https://doi.org/10.1177/1934578X1801300510
Wang ML, Jian YS, Wei JQ, Wei X, Qi XX, Jiang SY, Wang ZM (2008) Effects of irradiance on growth, photosynthetic characteristics, and artemisinin content of Artemisia annua L. Photosynthetica 46:17–20. https://doi.org/10.1007/s11099-008-0004-1
Zobayed SSPK, Saxena PK (2004) Production of St. John’s wort plants under controlled environment for maximizing biomass and secondary metabolites. In Vitro Cell Dev Biol Plant 40:108–114. https://doi.org/10.1079/IVP2003498
Acknowledgements
This work was supported by the “Industrial Core Technology Development Project (No. 10049146),” Ministry of Trade, Industry, and Energy, Republic of Korea.
Author information
Authors and Affiliations
Contributions
JWL, ZH, and JES designed the research; JWL and ZH performed the experiments; JWL, ZH, and WHK analyzed the results; and JWL and ZH prepared the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Sanghyun Lee.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lee, J.W., Han, Z., Kang, W.H. et al. Effects of seasonal light variation and artificial light treatments on growth and flavonoid production of Artemisia princeps cultivated in greenhouses. Hortic. Environ. Biotechnol. 62, 253–261 (2021). https://doi.org/10.1007/s13580-021-00335-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13580-021-00335-0