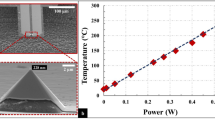
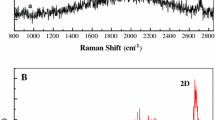
Abstract
A self-terminated electrochemical atomic layer deposition process is developed to fabricate Au monolayer (ML) film layer-by-layer. It is found that the under potential deposited hydrogen (Hupd) provides perfect termination after each ML deposition and the further ML growth can be replicated after a surface activation using a positive potential to remove the Hupd layer. Voltammetric measurements, deposition current analysis, and EQCM show clear characteristics of UPD hydrogen surface termination and the ML deposition. Both XRR and HREED confirm the Au ML film formation. Moreover, the Au ML film appears to be effective for surface enhanced Raman effect of GO on the Au ML film.






Similar content being viewed by others
References
Kale MJ, Avanesian T, Christopher P (2014) Direct photocatalysis by plasmonic nanostructures. ACS Catal 4:116–128. https://doi.org/10.1021/cs400993w
Huang L, Rudolph M, Rominger F, Hashmi ASK (2016) Photosensitizer-free visible-light-mediated gold-catalyzed 1, 2-difunctionalization of alkynes. Angew Chem Int Ed 55:4808–4813. https://doi.org/10.1002/anie.201511487
Witzel S, Xie J, Rudolph M, Hashmi ASK (2017) Photosensitizer-free, gold-catalyzed C–C cross-coupling of boronic acids and diazonium salts enabled by visible light. Adv Synth Catal 359:1522–1528. https://doi.org/10.1002/adsc.201700121
Xie J, Zhang T, Chen F, Mehrkens N, Rominger F, Rudolph M, Hashmi ASK (2016) Gold-catalyzed highly selective photoredox C(sp2)-H difluoroalkylation and perfluoroalkylation of hydrazones. Angew Chem Int Ed 55:2934–2938. https://doi.org/10.1002/anie.201508622
Haruta M, Yamada N, Kobayashi T, Iijima S (1989) Gold catalysts prepared by coprecipitation for low-temperature oxidation of hydrogen and of carbon monoxide. J Catal 115:301–309. https://doi.org/10.1016/0021-9517(89)90034-1
Saavedra J, Doan HA, Pursell CJ, Grabow LC, Chandler BD (2014) The critical role of water at the gold-titania interface in catalytic CO oxidation. Science 345:1599–1602. https://doi.org/10.1126/science.1256018
Mao K, Li L, Zhang W, Pei Y, Zeng XC, Wu X, Yang J (2014) A theoretical study of single-atom catalysis of CO oxidation using Au embedded 2D h-BN monolayer: a CO-promoted O2 activation. Sci Rep 4(5441). https://doi.org/10.1038/srep05441
Zhang H, Watanabe T, Okumura M, Haruta M, Toshima N (2012) Catalytically highly active top gold atom on palladium nanocluster. Nat Mater 11:49–52. https://doi.org/10.1038/nmat3143
Hughes MD, Xu Y-J, Jenkins P, McMorn P, Landon P, Enache DI, Carley AF, Attard GA, Hutchings GJ, King F, Stitt EH, Johnston P, Griffin K, Kiely CJ (2005) Tunable gold catalysts for selective hydrocarbon oxidation under mild conditions. Nature 437:1132–1135. https://doi.org/10.1038/nature04190
Hashmi ASK, Hutchings GJ (2006) Gold catalysis. Angew Chem Int Ed 45:7896–7936. https://doi.org/10.1002/anie.200602454
Banerjee A, Su T, Beglau D, Pietka G, Liu F, Almutawalli S, Yang J, Guha S (2012) High-efficiency, multijunction nc-Si:H-based solar cells at high deposition rate. IEEE J Photovolt 2:99–103. https://doi.org/10.1109/JPHOTOV.2011.2180892
Dong H, Zhang J, Ju H, Lu H, Wang S, Jin S, Hao K, Du H, Zhang X (2012) Highly sensitive multiple microRNA detection based on fluorescence quenching of graphene oxide and isothermal strand-displacement polymerase reaction. Anal Chem 84:4587–4593. https://doi.org/10.1021/ac300721u
Liu Y, Yu D, Zeng C, Miao Z, Dai L (2010) Biocompatible graphene oxide-based glucose biosensors. Langmuir 26:6158–6160. https://doi.org/10.1021/la100886x
Yu D, Park K, Durstock M, Dai L (2011) Fullerene-grafted graphene for efficient bulk heterojunction polymer photovoltaic devices. J Phys Chem Lett 2:1113–1118. https://doi.org/10.1021/jz200428y
Tassin P, Koschny T, Soukoulis CM (2013) Graphene for terahertz applications. Science 341:620–621. https://doi.org/10.1126/science.1242253
Qu L, Liu Y, Baek JB, Dai L (2010) Nitrogen-doped graphene as efficient metal-free electrocatalyst for oxygen reduction in fuel cells. ACS Nano 4:1321–1326. https://doi.org/10.1021/nn901850u
Yu D, Dai L (2010) Self-assembled graphene/carbon nanotube hybrid films for supercapacitors. J Phys Chem Lett 1:467–470. https://doi.org/10.1021/jz9003137
Geim AK, Grigorieva IV (2013) Van der Waals heterostructures. Nature 499:419–425. https://doi.org/10.1038/nature12385
Xie X, Qu L, Zhou C, Li Y, Zhu J, Bai H, Shi G, Dai L (2010) An asymmetrically surface-modified graphene film electrochemical actuator. ACS Nano 4:6050–6054. https://doi.org/10.1021/nn101563x
Sau TK, Murphy CJ (2004) Room temperature, high-yield synthesis of multiple shapes of gold nanoparticles in aqueous solution. J Am Chem Soc 126:8648–8649. https://doi.org/10.1021/ja047846d
Ruemmele JA, Hall WP, Ruvuna LK, Duyne RPV (2013) A localized surface plasmon resonance imaging instrument for multiplexed biosensing. Anal Chem 85:4560–4566. https://doi.org/10.1021/ac400192f
Zhang Z, Zhang L, Hedhili MN, Zhang H, Wang P (2013) Plasmonic gold nanocrystals coupled with photonic crystal seamlessly on TiO2 nanotube photoelectrodes for efficient visible light photoelectrochemical water splitting. Nano Lett 13:14–20. https://doi.org/10.1021/nl3029202
Daniel MC, Astruc D (2004) Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev 104:293–346. https://doi.org/10.1021/cr030698+
Jasuja K, Vikas B (2009) Implantation and growth of dendritic gold nanostructures on graphene derivatives: electrical property tailoring and Raman enhancement. ACS Nano 3:2358–2366. https://doi.org/10.1021/nn900504v
Kim F, Song JH, Yang P (2002) Photochemical synthesis of gold nanorods. J Am Chem Soc 124:14316–14317. https://doi.org/10.1021/ja028110o
Zhao Y, Chen G, Du Y, Xu J, Wu S, Qu Y, Zhu Y (2014) Plasmonic-enhanced Raman scattering of graphene on growth substrates and its application in SERS. Nano 6:13754–13760. https://doi.org/10.1039/C4NR04225E
Zhou X, Liu H, Yang L, Liu J (2013) SERS and OWGS detection of dynamic trapping molecular TNT based on a functional self-assembly Au monolayer film. Analyst 138:1858–1864. https://doi.org/10.1039/C3AN36683A
Zhou X, Zhou F, Liu H, Yang L, Liu J (2013) Assembly of polymer–gold nanostructures with high reproducibility into a monolayer film SERS substrate with 5 nm gaps for pesticide trace detection. Analyst 138:5832–5838. https://doi.org/10.1039/C3AN00914A
George SM (2010) Atomic layer deposition: an overview. Chem Rev 110:111–131. https://doi.org/10.1021/cr900056b
Feng S, Yang J, Liu M, Zhu H, Zhang J, Li G, Peng J, Liu Q (2012) CdS quantum dots sensitized TiO2 nanorod-array-film photoelectrode on FTO substrate by electrochemical atomic layer epitaxy method. Electrochim Acta 83:321–326. https://doi.org/10.1016/j.electacta.2012.07.130
Gregory BW, Suggs DW, Stickney JL (1991) Conditions for the deposition of CdTe by electrochemical atomic layer epitaxy. J Electrochem Soc 138:1279–1284. https://doi.org/10.1149/1.2085773
Liu Y, Gokcen D, Bertocci U, Moffat TP (2012) Self-terminating growth of platinum films by electrochemical deposition. Science 338:1327–1330. https://doi.org/10.1126/science.1228925
Slater JC (1964) Atomic radii in crystals. J Chem Phys 41:3199–3204. https://doi.org/10.1063/1.1725697
Ma Q, Zhu XJ, Zhang DD, Liu SZ (2014) Graphene oxide—a surprisingly good nucleation seed and adhesion promotion agent for one-step ZnO lithography and optoelectronic applications. J Mater Chem C 2:8956–8961. https://doi.org/10.1039/c4c01573h
Losurdo M, Yi C, Suvorova A, Rubanov S, Kim T-H, Giangregorio MM, Jiao W, Bergmair I, Bruno G, Brown AS (2014) Demonstrating the capability of the high-performance plasmonic gallium–graphene couple. ACS Nano 8:3031–3041. https://doi.org/10.1021/nn500472r
Wang M, Han J, Xiong H, Guo R, Yin Y (2015) Nanostructured hybrid shells of r-GO/AuNP/m-TiO2 as highly active photocatalysts. ACS Appl Mater Interfaces 7:6909–6918. https://doi.org/10.1021/acsami.5b00663
Funding
This study was supported by the National Natural Science Foundation of China (51673157, 21706218), the National Natural Science Foundation of Shaanxi (2017JQ2013), the Scientific Research Foundation of Education Department of Shaanxi Provincial Government, China (17JK1164), High-level Talent Research Fund of Xijing University (XJ17T01), and Scientific Research Project of Sichuan University of Science and Engineering (2017RCL70).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ma, Q., Ren, X., Pang, L. et al. Au monolayer film coating with graphene oxide for surface enhanced Raman effect. Gold Bull 51, 27–33 (2018). https://doi.org/10.1007/s13404-018-0226-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13404-018-0226-3